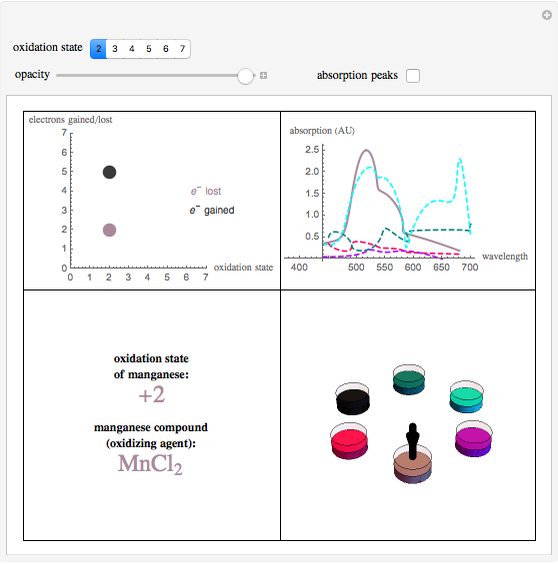
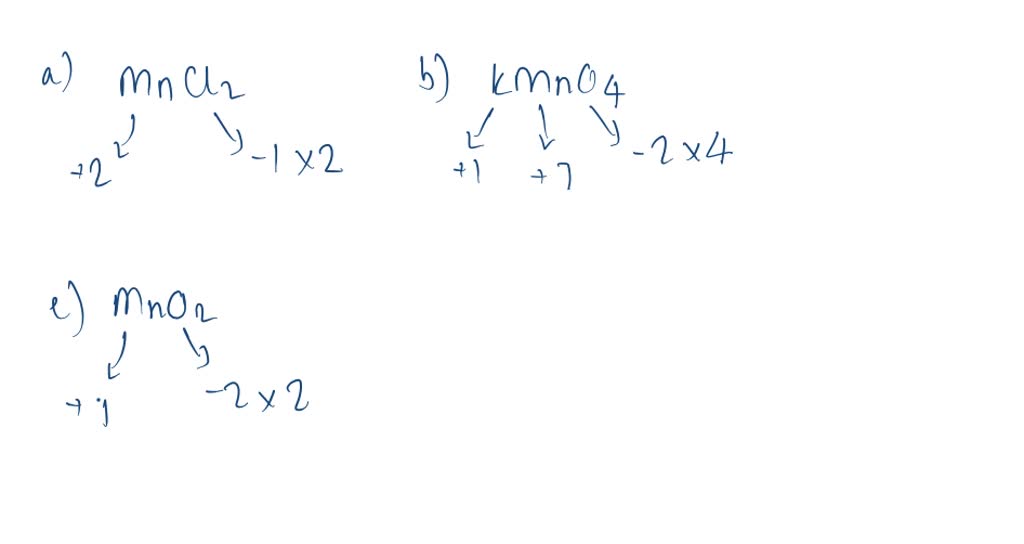What Is The Oxidation State Of Manganese In Kmno4 - The oxidation number of manganese in kmno₄ is +7. Thus, we have an equation: One potassium atom, one manganese atom, and four oxygen atoms make up potassium. Let the oxidation state of manganese be ‘x’. The oxidation state of manganese in potassium permanganate (kmno4) is +7.
The oxidation state of manganese in potassium permanganate (kmno4) is +7. Let the oxidation state of manganese be ‘x’. Thus, we have an equation: One potassium atom, one manganese atom, and four oxygen atoms make up potassium. The oxidation number of manganese in kmno₄ is +7.
Let the oxidation state of manganese be ‘x’. The oxidation number of manganese in kmno₄ is +7. The oxidation state of manganese in potassium permanganate (kmno4) is +7. One potassium atom, one manganese atom, and four oxygen atoms make up potassium. Thus, we have an equation:
Manganese achieves its highest oxidation state in its compound (a) MnO2
The oxidation number of manganese in kmno₄ is +7. Let the oxidation state of manganese be ‘x’. One potassium atom, one manganese atom, and four oxygen atoms make up potassium. The oxidation state of manganese in potassium permanganate (kmno4) is +7. Thus, we have an equation:
Manganese Oxidation States Manganese Molecules
Let the oxidation state of manganese be ‘x’. Thus, we have an equation: The oxidation number of manganese in kmno₄ is +7. The oxidation state of manganese in potassium permanganate (kmno4) is +7. One potassium atom, one manganese atom, and four oxygen atoms make up potassium.
SOLVED Glucose decolorizes KMnO4 because it Select one a. oxidizes the
One potassium atom, one manganese atom, and four oxygen atoms make up potassium. The oxidation number of manganese in kmno₄ is +7. Thus, we have an equation: Let the oxidation state of manganese be ‘x’. The oxidation state of manganese in potassium permanganate (kmno4) is +7.
Answered 4 WHAT IS THE MANGANESE OXIDATION STATE… bartleby
The oxidation number of manganese in kmno₄ is +7. Let the oxidation state of manganese be ‘x’. One potassium atom, one manganese atom, and four oxygen atoms make up potassium. Thus, we have an equation: The oxidation state of manganese in potassium permanganate (kmno4) is +7.
What Is the Oxidation State of Manganese in Kmno4 ElainehasAtkins
One potassium atom, one manganese atom, and four oxygen atoms make up potassium. Thus, we have an equation: The oxidation number of manganese in kmno₄ is +7. The oxidation state of manganese in potassium permanganate (kmno4) is +7. Let the oxidation state of manganese be ‘x’.
Manganese Oxidation States Wolfram Demonstrations Project
The oxidation state of manganese in potassium permanganate (kmno4) is +7. Thus, we have an equation: Let the oxidation state of manganese be ‘x’. The oxidation number of manganese in kmno₄ is +7. One potassium atom, one manganese atom, and four oxygen atoms make up potassium.
Manganese exhibits maximum oxidation state in Filo
Let the oxidation state of manganese be ‘x’. The oxidation state of manganese in potassium permanganate (kmno4) is +7. The oxidation number of manganese in kmno₄ is +7. Thus, we have an equation: One potassium atom, one manganese atom, and four oxygen atoms make up potassium.
OXIDATION STATES OF MANGANESE
Thus, we have an equation: Let the oxidation state of manganese be ‘x’. One potassium atom, one manganese atom, and four oxygen atoms make up potassium. The oxidation number of manganese in kmno₄ is +7. The oxidation state of manganese in potassium permanganate (kmno4) is +7.
Manganese Oxidation States Stock Image C028/0891 Science Photo
Thus, we have an equation: The oxidation number of manganese in kmno₄ is +7. One potassium atom, one manganese atom, and four oxygen atoms make up potassium. The oxidation state of manganese in potassium permanganate (kmno4) is +7. Let the oxidation state of manganese be ‘x’.
SOLVEDWhat is the oxidation state of manganese in each of the
Thus, we have an equation: The oxidation state of manganese in potassium permanganate (kmno4) is +7. One potassium atom, one manganese atom, and four oxygen atoms make up potassium. Let the oxidation state of manganese be ‘x’. The oxidation number of manganese in kmno₄ is +7.
Thus, We Have An Equation:
One potassium atom, one manganese atom, and four oxygen atoms make up potassium. Let the oxidation state of manganese be ‘x’. The oxidation number of manganese in kmno₄ is +7. The oxidation state of manganese in potassium permanganate (kmno4) is +7.