What Is The Oxidation Number Of Manganese In Mno4 - In the same vein, one might wonder. So, the oxidation number of manganese (mn) in potassium permanganate mno 2 is + 4. Click here👆to get an answer to your question ️ what is the oxidation number of manganese in. Let the oxidation state of mn be x.
In the same vein, one might wonder. Let the oxidation state of mn be x. So, the oxidation number of manganese (mn) in potassium permanganate mno 2 is + 4. Click here👆to get an answer to your question ️ what is the oxidation number of manganese in.
Let the oxidation state of mn be x. Click here👆to get an answer to your question ️ what is the oxidation number of manganese in. So, the oxidation number of manganese (mn) in potassium permanganate mno 2 is + 4. In the same vein, one might wonder.
(a) Average manganese oxidation number (AMON) for δMnO2, δMnO2** [15
Click here👆to get an answer to your question ️ what is the oxidation number of manganese in. Let the oxidation state of mn be x. So, the oxidation number of manganese (mn) in potassium permanganate mno 2 is + 4. In the same vein, one might wonder.
SOLVED The oxidation number of the manganese atom in MnO4 is
Click here👆to get an answer to your question ️ what is the oxidation number of manganese in. So, the oxidation number of manganese (mn) in potassium permanganate mno 2 is + 4. In the same vein, one might wonder. Let the oxidation state of mn be x.
manganese oxidation Oxidation Technologies News
So, the oxidation number of manganese (mn) in potassium permanganate mno 2 is + 4. Let the oxidation state of mn be x. In the same vein, one might wonder. Click here👆to get an answer to your question ️ what is the oxidation number of manganese in.
SOLVED When MnO4 reacts to form Mn2+, the manganese in MnO4 is
Click here👆to get an answer to your question ️ what is the oxidation number of manganese in. In the same vein, one might wonder. So, the oxidation number of manganese (mn) in potassium permanganate mno 2 is + 4. Let the oxidation state of mn be x.
SOLVED The oxidation number of the manganese atom in MnO4 is
Let the oxidation state of mn be x. In the same vein, one might wonder. Click here👆to get an answer to your question ️ what is the oxidation number of manganese in. So, the oxidation number of manganese (mn) in potassium permanganate mno 2 is + 4.
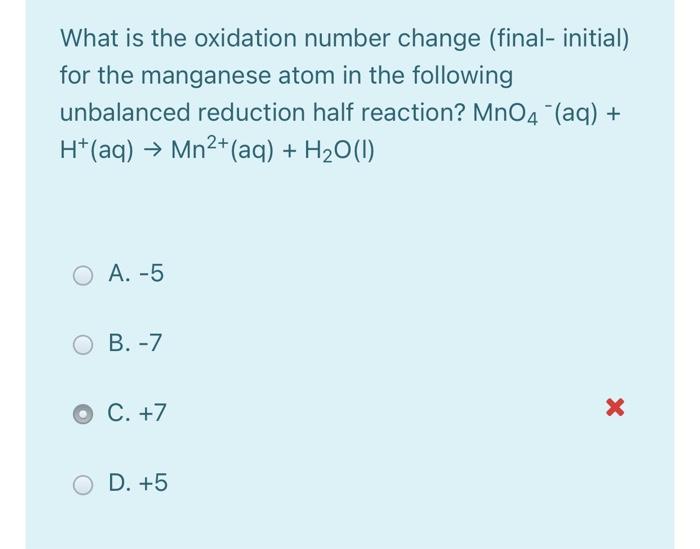
Solved What is the oxidation number change (final initial)
So, the oxidation number of manganese (mn) in potassium permanganate mno 2 is + 4. Click here👆to get an answer to your question ️ what is the oxidation number of manganese in. Let the oxidation state of mn be x. In the same vein, one might wonder.
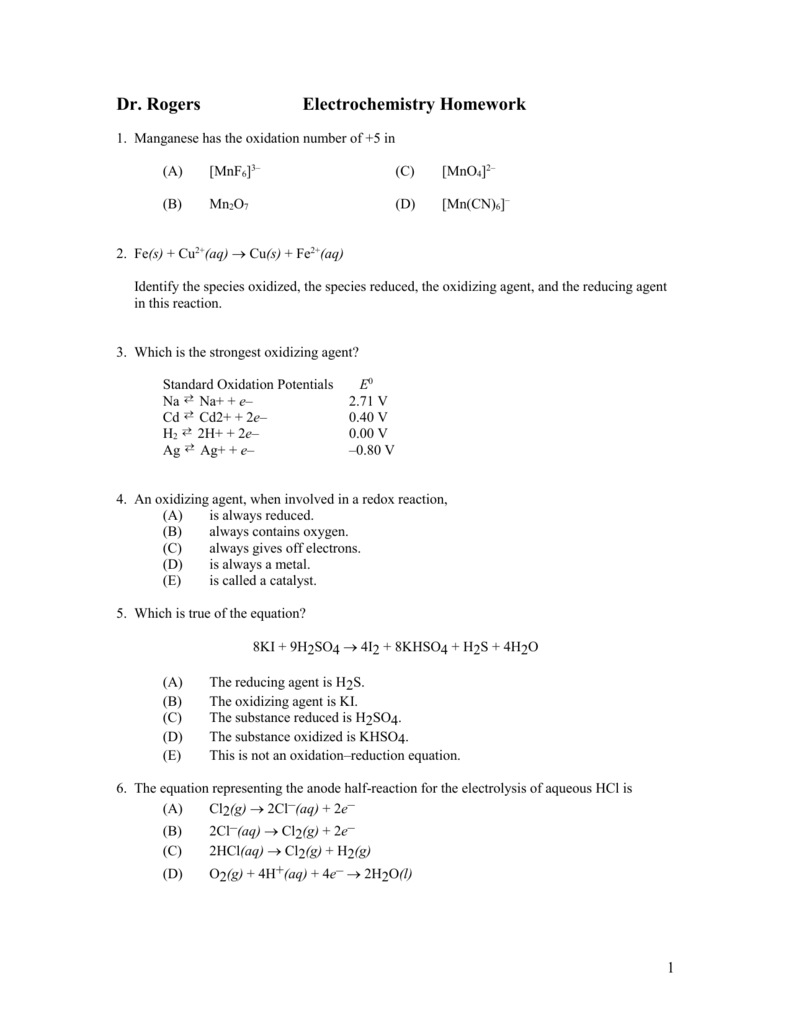
Manganese has the oxidation number of +5 in
So, the oxidation number of manganese (mn) in potassium permanganate mno 2 is + 4. Let the oxidation state of mn be x. In the same vein, one might wonder. Click here👆to get an answer to your question ️ what is the oxidation number of manganese in.
Solved Manganese has the oxidation number of +7 in X A.
Click here👆to get an answer to your question ️ what is the oxidation number of manganese in. So, the oxidation number of manganese (mn) in potassium permanganate mno 2 is + 4. In the same vein, one might wonder. Let the oxidation state of mn be x.
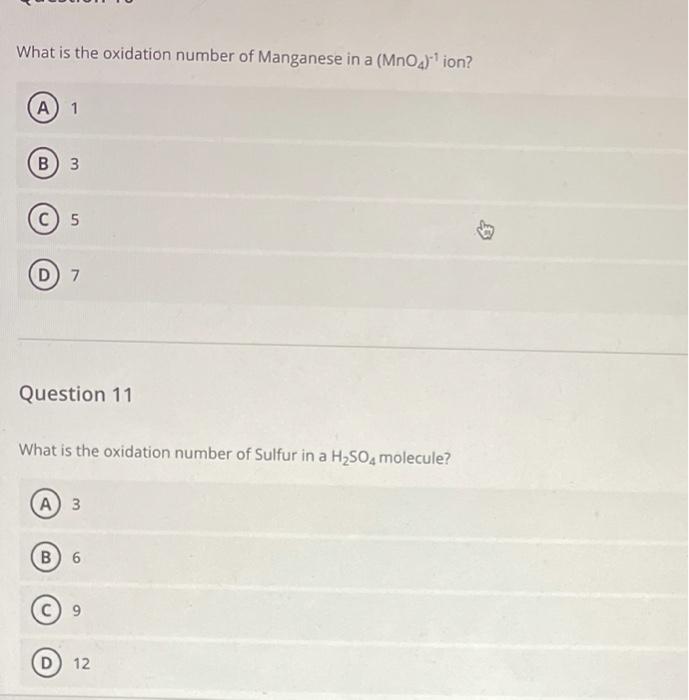
Solved What is the oxidation number of Manganese in a (MnO4)
In the same vein, one might wonder. So, the oxidation number of manganese (mn) in potassium permanganate mno 2 is + 4. Click here👆to get an answer to your question ️ what is the oxidation number of manganese in. Let the oxidation state of mn be x.
SOLVED Manganese has the oxidation number of +7 in OA [MnF6)]3. B
So, the oxidation number of manganese (mn) in potassium permanganate mno 2 is + 4. Click here👆to get an answer to your question ️ what is the oxidation number of manganese in. In the same vein, one might wonder. Let the oxidation state of mn be x.
Let The Oxidation State Of Mn Be X.
Click here👆to get an answer to your question ️ what is the oxidation number of manganese in. In the same vein, one might wonder. So, the oxidation number of manganese (mn) in potassium permanganate mno 2 is + 4.



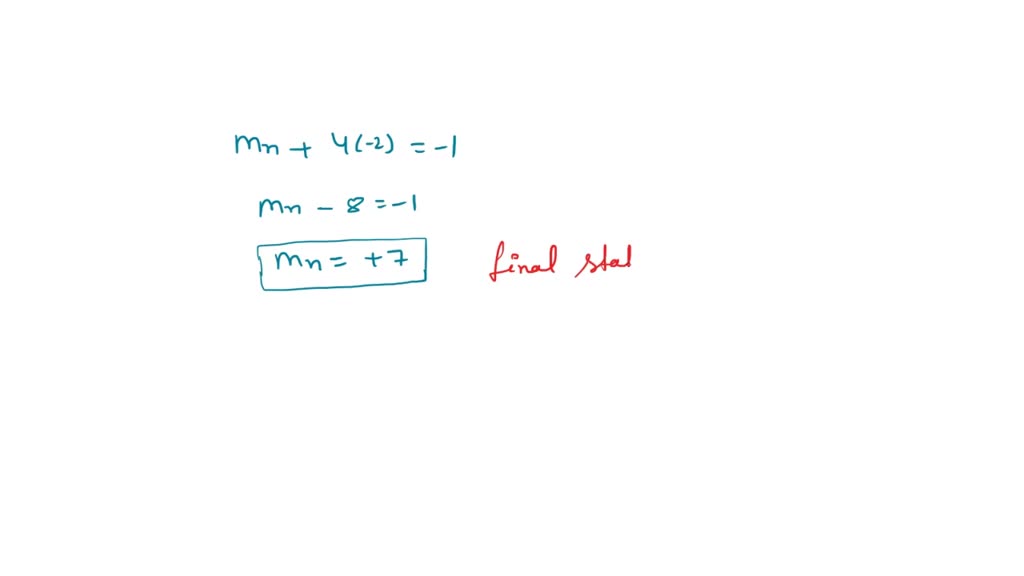


![SOLVED Manganese has the oxidation number of +7 in OA [MnF6)]3. B](https://cdn.numerade.com/ask_images/eefa9df997f84c96ba55933c7a65b854.jpg)