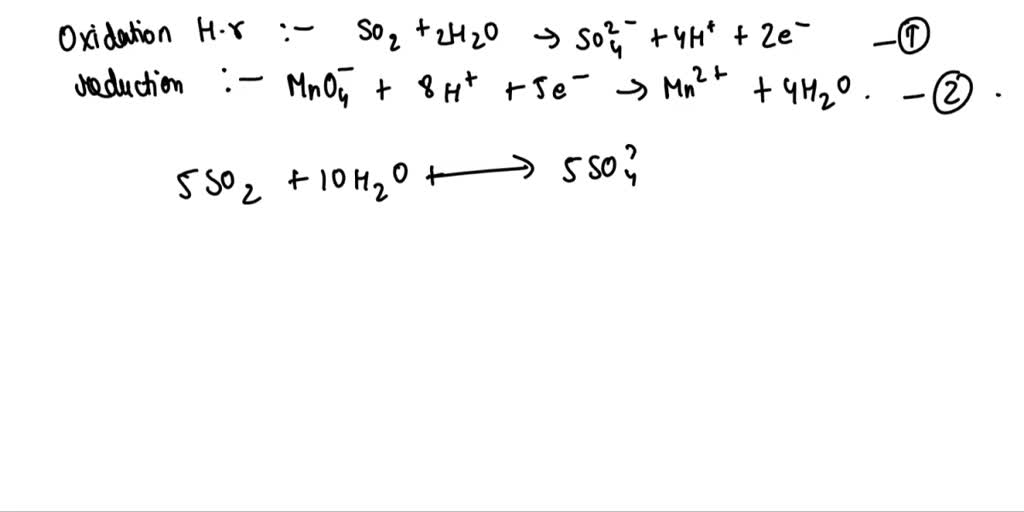What Is The Oxidation Number Of Manganese In Mno2 - The aggregate number of electrons that an atom either absorbs or loses in order to form a. Since the sum of the oxidation numbers must be $$ 0 $$, the oxidation number of the. Since the sum of the oxidation numbers in a neutral compound is zero, the oxidation number. Calculating the oxidation number of manganese(mn) in mno 2: The oxidation number of the manganese ion in the compound with the.
Calculating the oxidation number of manganese(mn) in mno 2: The aggregate number of electrons that an atom either absorbs or loses in order to form a. Since the sum of the oxidation numbers in a neutral compound is zero, the oxidation number. Since the sum of the oxidation numbers must be $$ 0 $$, the oxidation number of the. The oxidation number of the manganese ion in the compound with the.
Calculating the oxidation number of manganese(mn) in mno 2: The oxidation number of the manganese ion in the compound with the. Since the sum of the oxidation numbers in a neutral compound is zero, the oxidation number. The aggregate number of electrons that an atom either absorbs or loses in order to form a. Since the sum of the oxidation numbers must be $$ 0 $$, the oxidation number of the.
(a) Average manganese oxidation number (AMON) for δMnO2, δMnO2** [15
Calculating the oxidation number of manganese(mn) in mno 2: The oxidation number of the manganese ion in the compound with the. Since the sum of the oxidation numbers in a neutral compound is zero, the oxidation number. The aggregate number of electrons that an atom either absorbs or loses in order to form a. Since the sum of the oxidation.
(a) Average manganese oxidation number (AMON) for δMnO2, δMnO2** [15
Since the sum of the oxidation numbers must be $$ 0 $$, the oxidation number of the. Calculating the oxidation number of manganese(mn) in mno 2: Since the sum of the oxidation numbers in a neutral compound is zero, the oxidation number. The aggregate number of electrons that an atom either absorbs or loses in order to form a. The.
Determine the oxidation number of manganese in the products as per the
Since the sum of the oxidation numbers in a neutral compound is zero, the oxidation number. Since the sum of the oxidation numbers must be $$ 0 $$, the oxidation number of the. Calculating the oxidation number of manganese(mn) in mno 2: The aggregate number of electrons that an atom either absorbs or loses in order to form a. The.
OXIDATION STATES OF MANGANESE
Since the sum of the oxidation numbers must be $$ 0 $$, the oxidation number of the. The aggregate number of electrons that an atom either absorbs or loses in order to form a. Since the sum of the oxidation numbers in a neutral compound is zero, the oxidation number. The oxidation number of the manganese ion in the compound.
SOLVED what is the oxidation number of nitrogen in manganese in MnO2?
Calculating the oxidation number of manganese(mn) in mno 2: The aggregate number of electrons that an atom either absorbs or loses in order to form a. Since the sum of the oxidation numbers must be $$ 0 $$, the oxidation number of the. Since the sum of the oxidation numbers in a neutral compound is zero, the oxidation number. The.
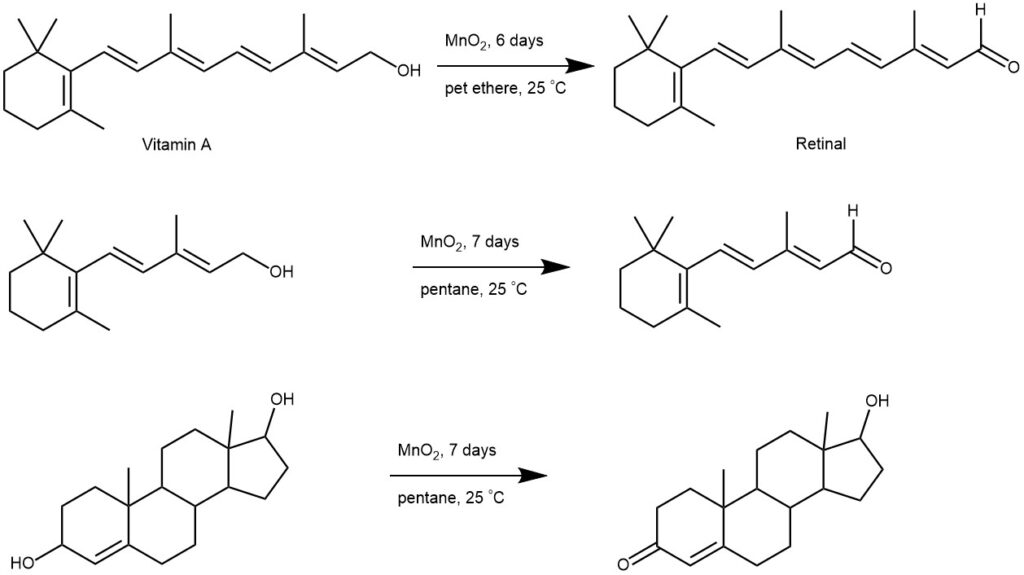
OXIDATION OF ALCOHOL BY MANGANESE DIOXIDE (MnO2) My chemistry blog
Calculating the oxidation number of manganese(mn) in mno 2: Since the sum of the oxidation numbers must be $$ 0 $$, the oxidation number of the. The oxidation number of the manganese ion in the compound with the. Since the sum of the oxidation numbers in a neutral compound is zero, the oxidation number. The aggregate number of electrons that.
Solved Manganese has the oxidation number of +7 in X A.
Since the sum of the oxidation numbers must be $$ 0 $$, the oxidation number of the. Calculating the oxidation number of manganese(mn) in mno 2: The oxidation number of the manganese ion in the compound with the. Since the sum of the oxidation numbers in a neutral compound is zero, the oxidation number. The aggregate number of electrons that.
(a) Average manganese oxidation number (AMON) for δMnO2, δMnO2** [15
The oxidation number of the manganese ion in the compound with the. Since the sum of the oxidation numbers must be $$ 0 $$, the oxidation number of the. Since the sum of the oxidation numbers in a neutral compound is zero, the oxidation number. The aggregate number of electrons that an atom either absorbs or loses in order to.
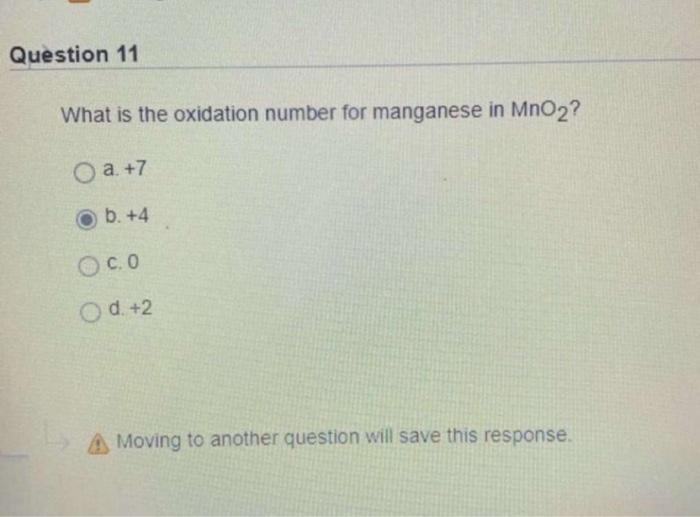
Solved Question 11 What is the oxidation number for
Calculating the oxidation number of manganese(mn) in mno 2: The oxidation number of the manganese ion in the compound with the. Since the sum of the oxidation numbers must be $$ 0 $$, the oxidation number of the. Since the sum of the oxidation numbers in a neutral compound is zero, the oxidation number. The aggregate number of electrons that.
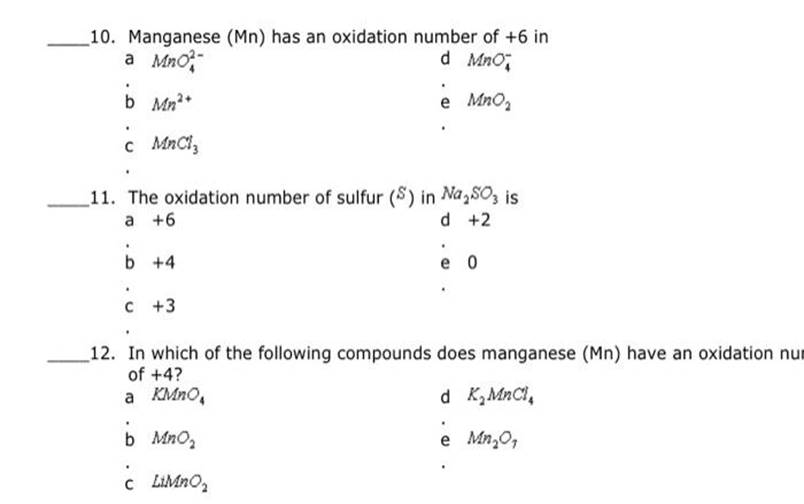
(Get Answer) 10. Manganese (Mn) Has An Oxidation Number Of +6 In A
The oxidation number of the manganese ion in the compound with the. Calculating the oxidation number of manganese(mn) in mno 2: Since the sum of the oxidation numbers in a neutral compound is zero, the oxidation number. Since the sum of the oxidation numbers must be $$ 0 $$, the oxidation number of the. The aggregate number of electrons that.
The Aggregate Number Of Electrons That An Atom Either Absorbs Or Loses In Order To Form A.
Since the sum of the oxidation numbers must be $$ 0 $$, the oxidation number of the. The oxidation number of the manganese ion in the compound with the. Since the sum of the oxidation numbers in a neutral compound is zero, the oxidation number. Calculating the oxidation number of manganese(mn) in mno 2: