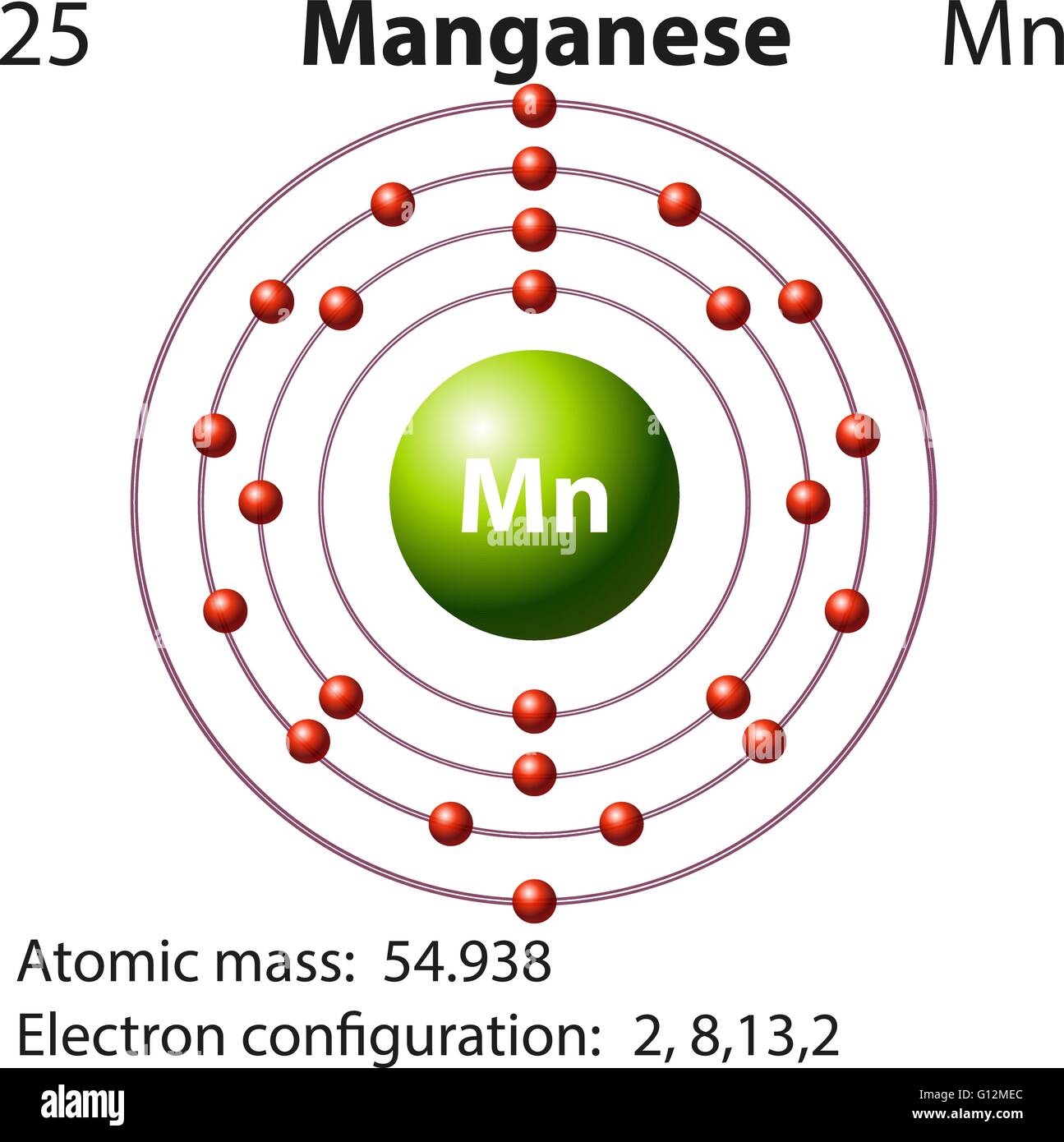
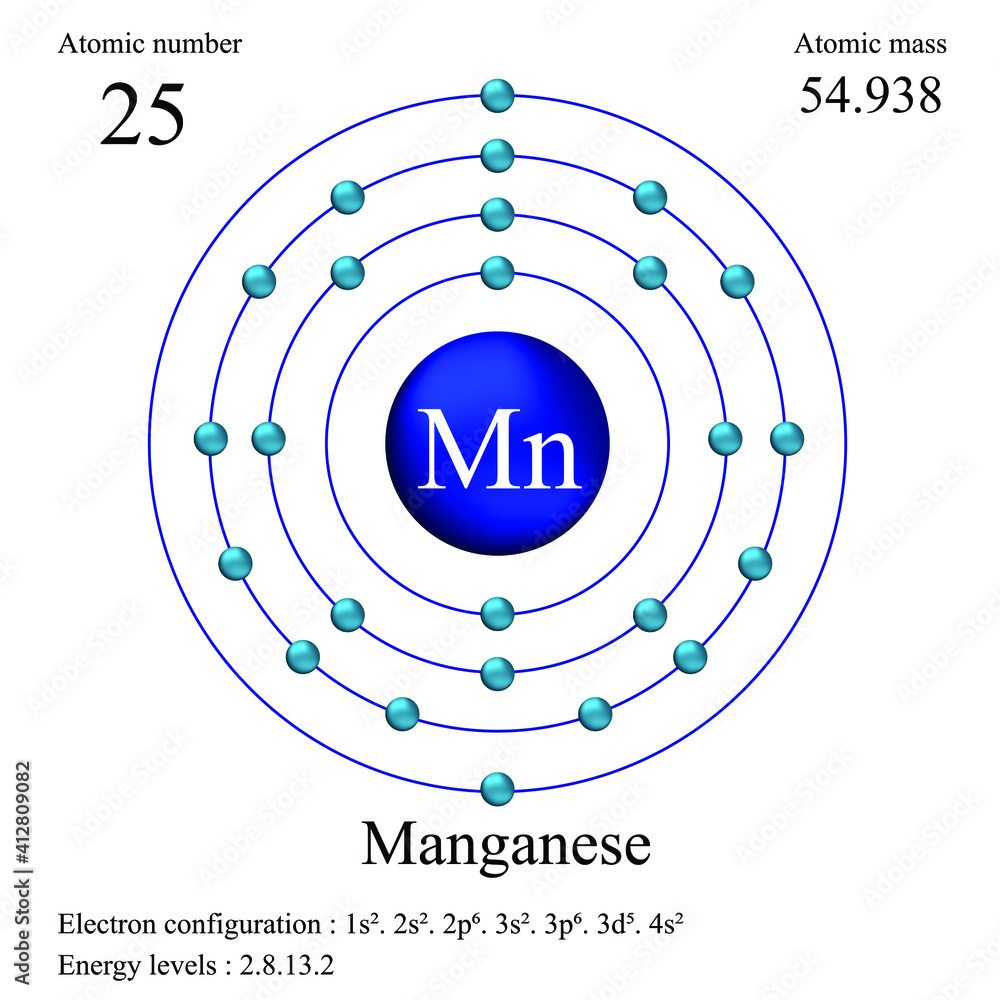
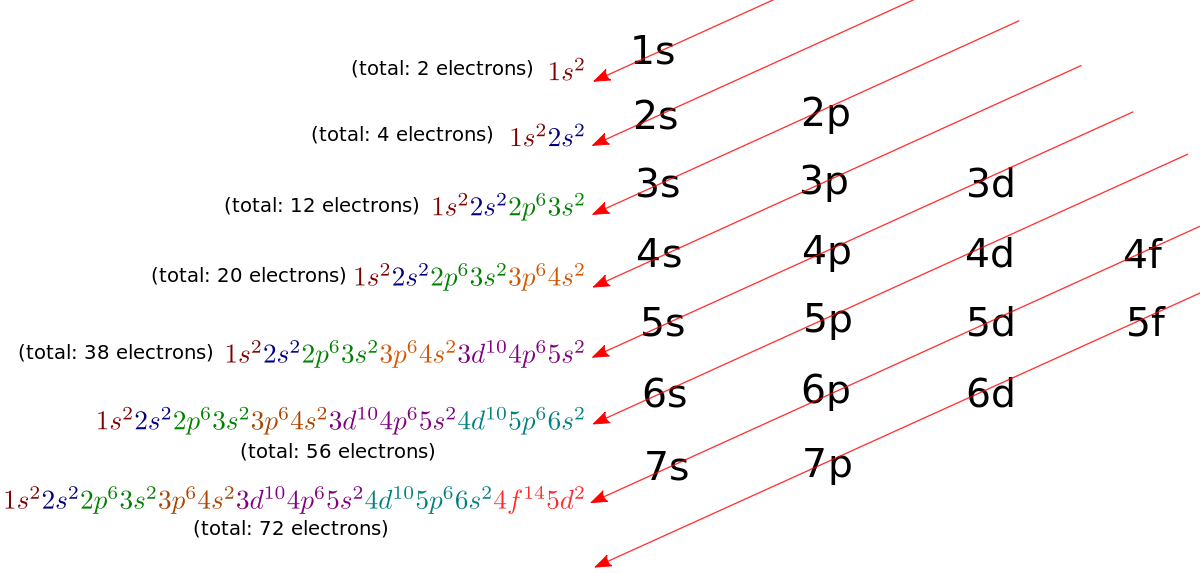
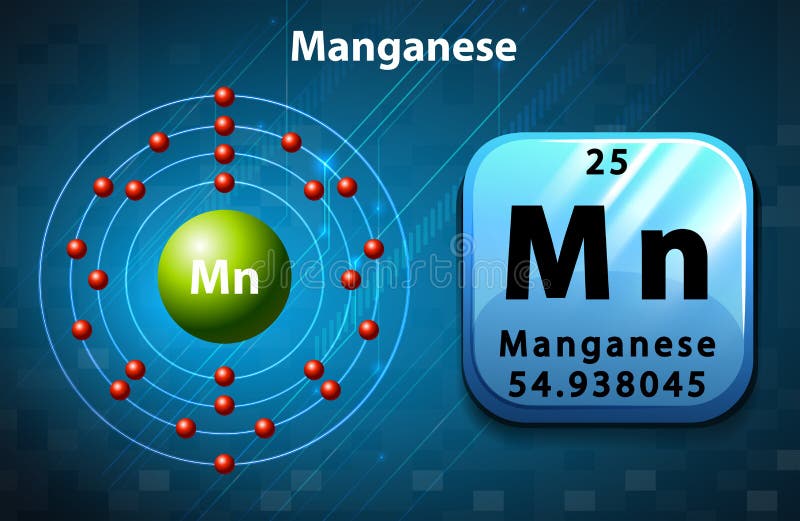
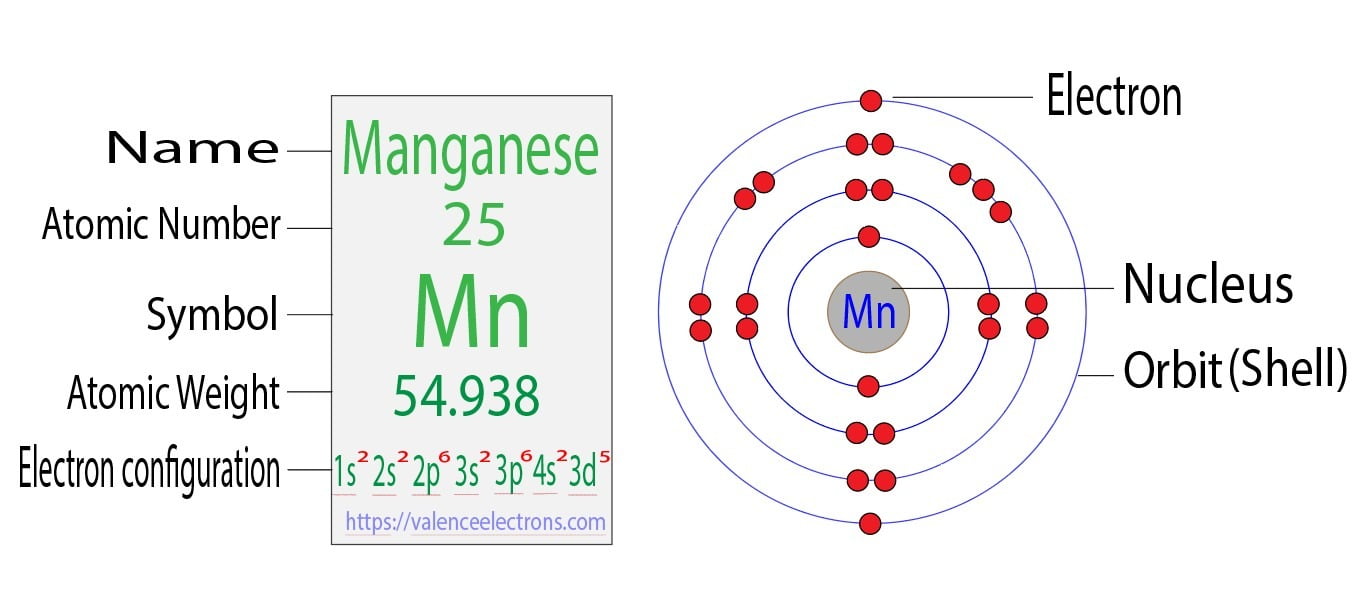
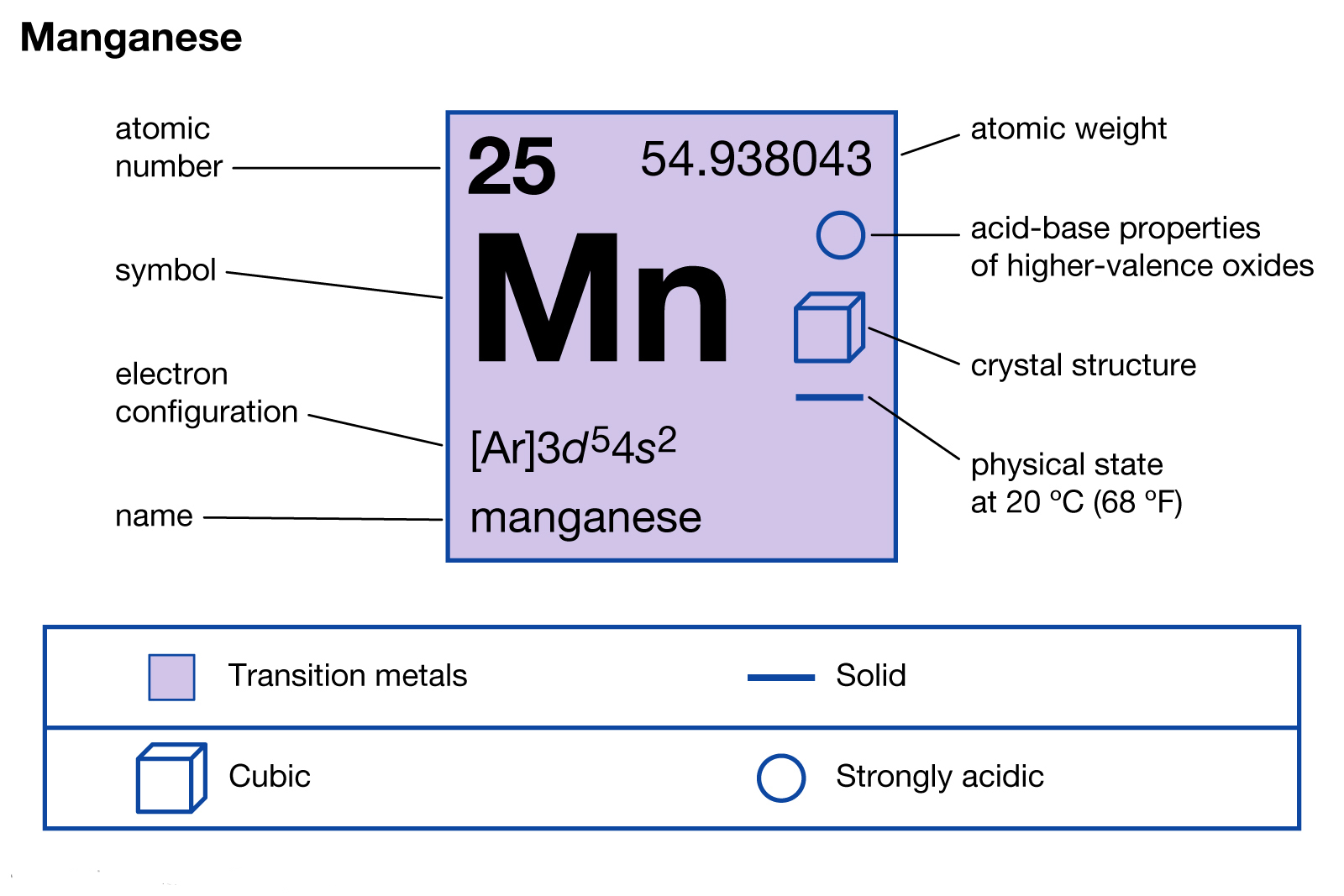
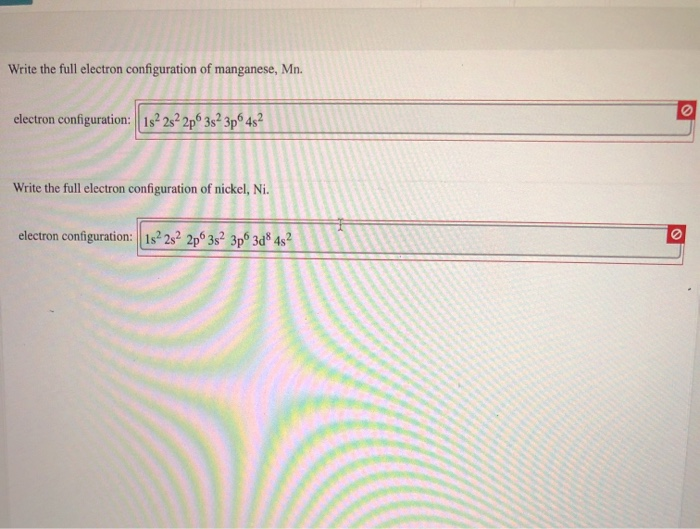
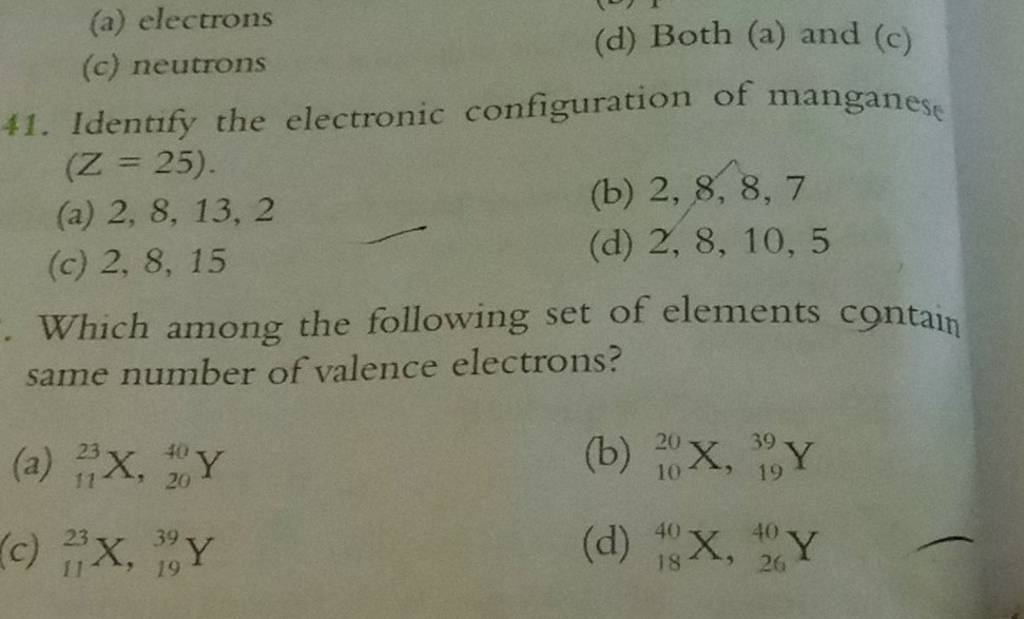What Is The Electronic Configuration Of Manganese - Mn (manganese) is an element with position number 25 in the periodic table. The electron configuration of manganese refers to the arrangement of electrons in the manganese. Thus its electronic configuration is [a r] 4 s 2 3. The atomic number of manganese (mn) is 25.
Mn (manganese) is an element with position number 25 in the periodic table. Thus its electronic configuration is [a r] 4 s 2 3. The atomic number of manganese (mn) is 25. The electron configuration of manganese refers to the arrangement of electrons in the manganese.
Mn (manganese) is an element with position number 25 in the periodic table. The atomic number of manganese (mn) is 25. Thus its electronic configuration is [a r] 4 s 2 3. The electron configuration of manganese refers to the arrangement of electrons in the manganese.
Electron Configuration Of Manganese Long Form
The atomic number of manganese (mn) is 25. Thus its electronic configuration is [a r] 4 s 2 3. The electron configuration of manganese refers to the arrangement of electrons in the manganese. Mn (manganese) is an element with position number 25 in the periodic table.
Manganese atomic structure has atomic number, atomic mass, electron
Mn (manganese) is an element with position number 25 in the periodic table. Thus its electronic configuration is [a r] 4 s 2 3. The atomic number of manganese (mn) is 25. The electron configuration of manganese refers to the arrangement of electrons in the manganese.
Electronic Configuration Of Manganese
The atomic number of manganese (mn) is 25. Mn (manganese) is an element with position number 25 in the periodic table. The electron configuration of manganese refers to the arrangement of electrons in the manganese. Thus its electronic configuration is [a r] 4 s 2 3.
Electron Configuration Of Manganese
Mn (manganese) is an element with position number 25 in the periodic table. The electron configuration of manganese refers to the arrangement of electrons in the manganese. The atomic number of manganese (mn) is 25. Thus its electronic configuration is [a r] 4 s 2 3.
Manganese Bohr Model
The atomic number of manganese (mn) is 25. Mn (manganese) is an element with position number 25 in the periodic table. Thus its electronic configuration is [a r] 4 s 2 3. The electron configuration of manganese refers to the arrangement of electrons in the manganese.
Manganese Electron Configuration Dynamic Periodic Table of Elements
Thus its electronic configuration is [a r] 4 s 2 3. The atomic number of manganese (mn) is 25. The electron configuration of manganese refers to the arrangement of electrons in the manganese. Mn (manganese) is an element with position number 25 in the periodic table.
Draw The Electron Configuration For A Neutral Atom Of Manganese
The atomic number of manganese (mn) is 25. The electron configuration of manganese refers to the arrangement of electrons in the manganese. Mn (manganese) is an element with position number 25 in the periodic table. Thus its electronic configuration is [a r] 4 s 2 3.
Solved Write the full electron configuration of manganese,
The electron configuration of manganese refers to the arrangement of electrons in the manganese. Thus its electronic configuration is [a r] 4 s 2 3. The atomic number of manganese (mn) is 25. Mn (manganese) is an element with position number 25 in the periodic table.
Identify the electronic configuration of manganese (Z=25). Filo
The electron configuration of manganese refers to the arrangement of electrons in the manganese. The atomic number of manganese (mn) is 25. Thus its electronic configuration is [a r] 4 s 2 3. Mn (manganese) is an element with position number 25 in the periodic table.
Full Electron Configuration Of Manganese
Mn (manganese) is an element with position number 25 in the periodic table. The atomic number of manganese (mn) is 25. The electron configuration of manganese refers to the arrangement of electrons in the manganese. Thus its electronic configuration is [a r] 4 s 2 3.
The Atomic Number Of Manganese (Mn) Is 25.
The electron configuration of manganese refers to the arrangement of electrons in the manganese. Thus its electronic configuration is [a r] 4 s 2 3. Mn (manganese) is an element with position number 25 in the periodic table.