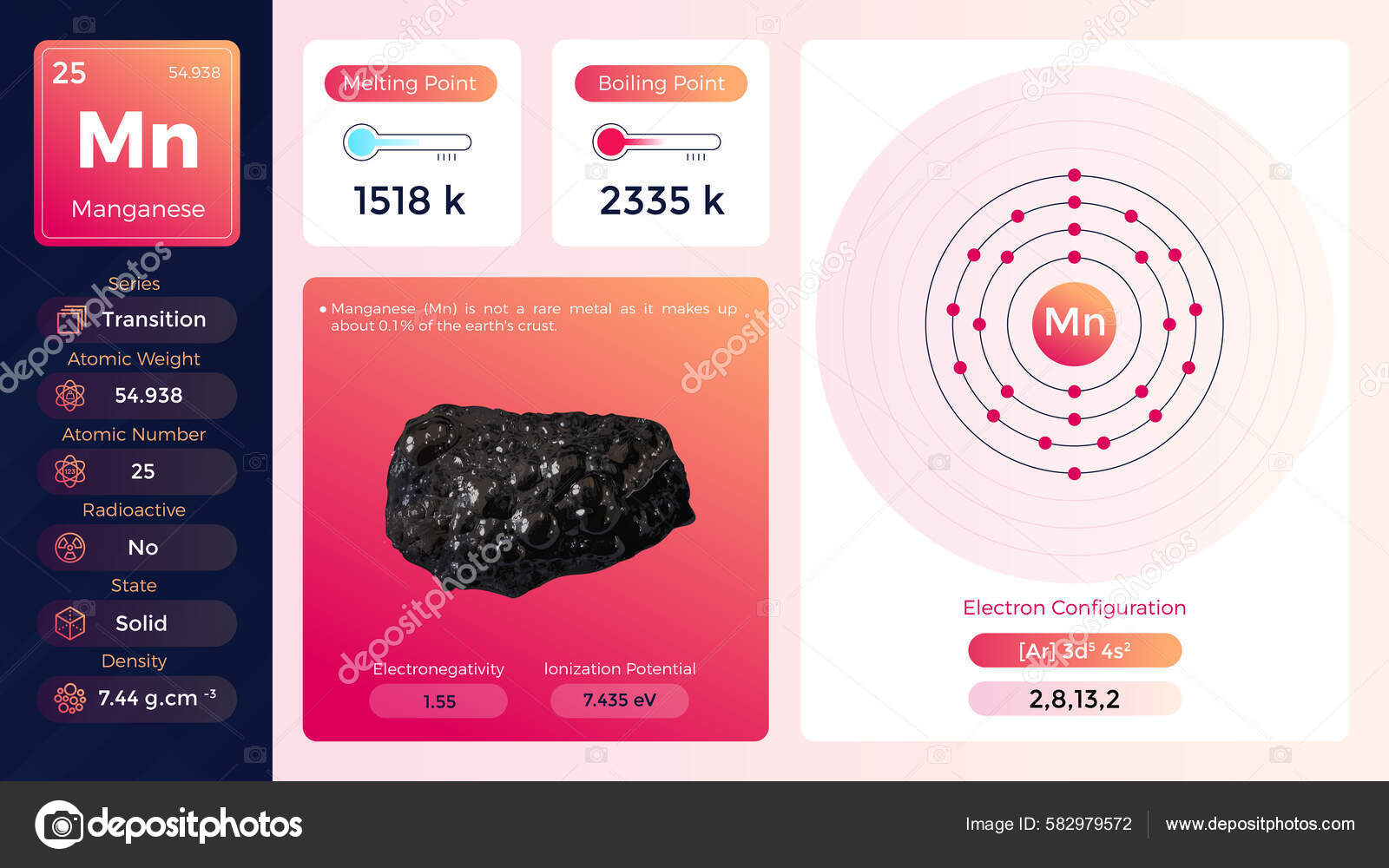
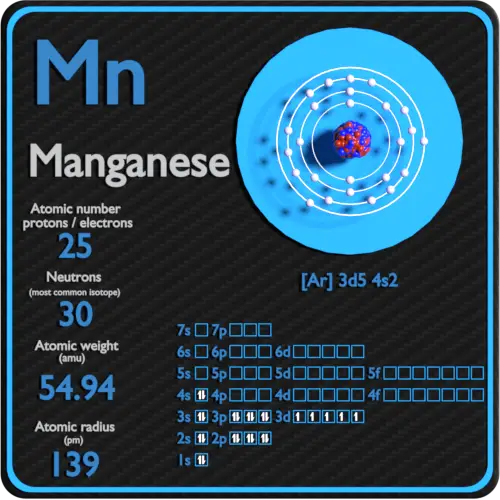
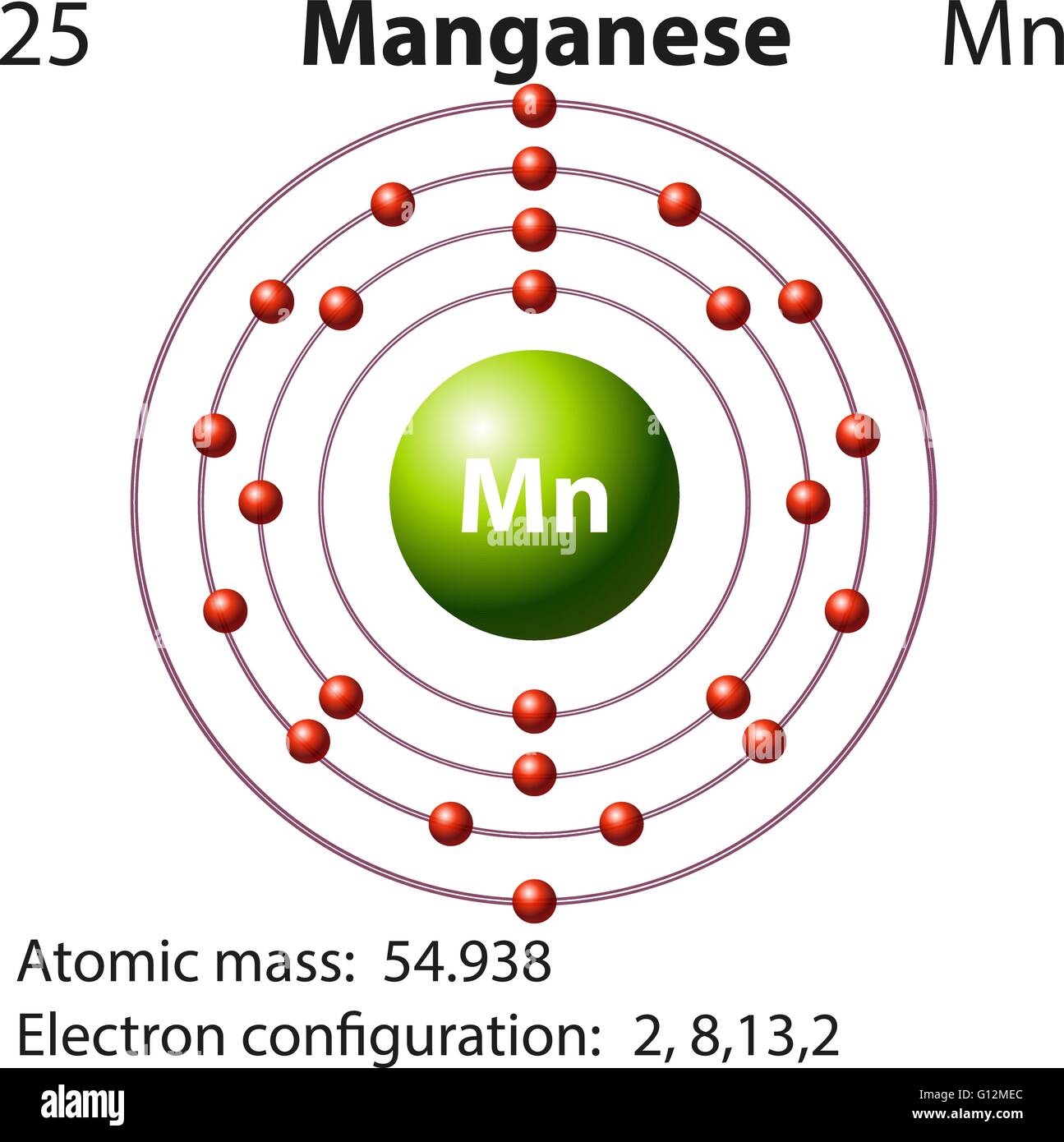
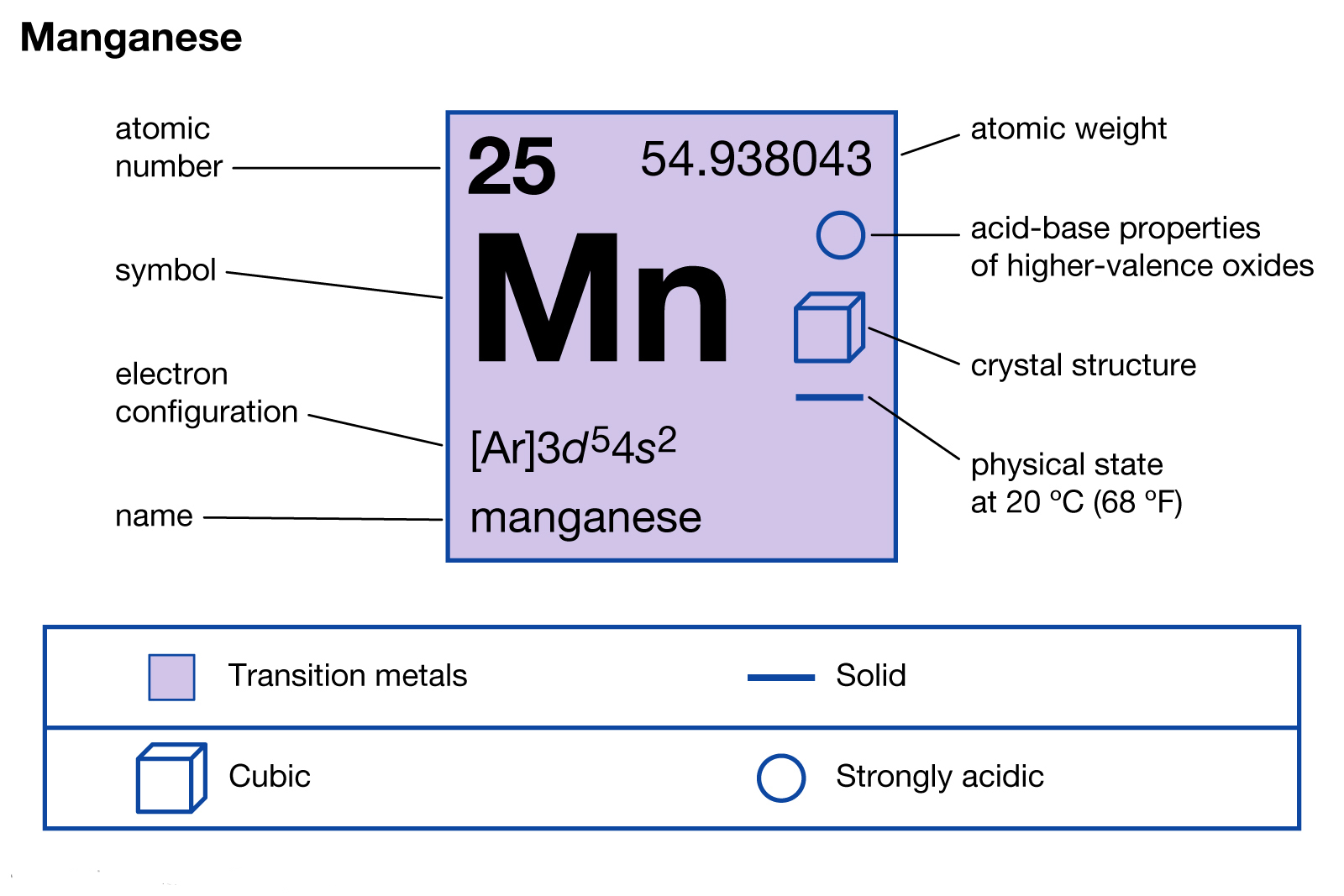
Manganese Electron Configuration Long Form - Calculate the effective nuclear charge of the last electron in an atom whose configuration is 1s22s22p63s23p5. Manganese atoms have 25 electrons and the shell structure is 2.8.13.2. Mn (manganese) is an element with position number 25 in the periodic table. Located in the iv period. The ground state electron configuration of ground state. The full electron configuration of this element is 1s2 2s2 2p6 3s2 3p6 3d5 4s2, while its shortened. The following table lists the ionization energies ie (ionization potentials);. Shorthand electron configuration of manganese: The complete electron configuration for manganese should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d5 and the abbreviated electron.
The full electron configuration of this element is 1s2 2s2 2p6 3s2 3p6 3d5 4s2, while its shortened. Shorthand electron configuration of manganese: Mn (manganese) is an element with position number 25 in the periodic table. The following table lists the ionization energies ie (ionization potentials);. The complete electron configuration for manganese should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d5 and the abbreviated electron. The ground state electron configuration of ground state. Manganese atoms have 25 electrons and the shell structure is 2.8.13.2. Located in the iv period. Calculate the effective nuclear charge of the last electron in an atom whose configuration is 1s22s22p63s23p5.
The complete electron configuration for manganese should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d5 and the abbreviated electron. Shorthand electron configuration of manganese: Mn (manganese) is an element with position number 25 in the periodic table. Calculate the effective nuclear charge of the last electron in an atom whose configuration is 1s22s22p63s23p5. The following table lists the ionization energies ie (ionization potentials);. The full electron configuration of this element is 1s2 2s2 2p6 3s2 3p6 3d5 4s2, while its shortened. The ground state electron configuration of ground state. Manganese atoms have 25 electrons and the shell structure is 2.8.13.2. Located in the iv period.
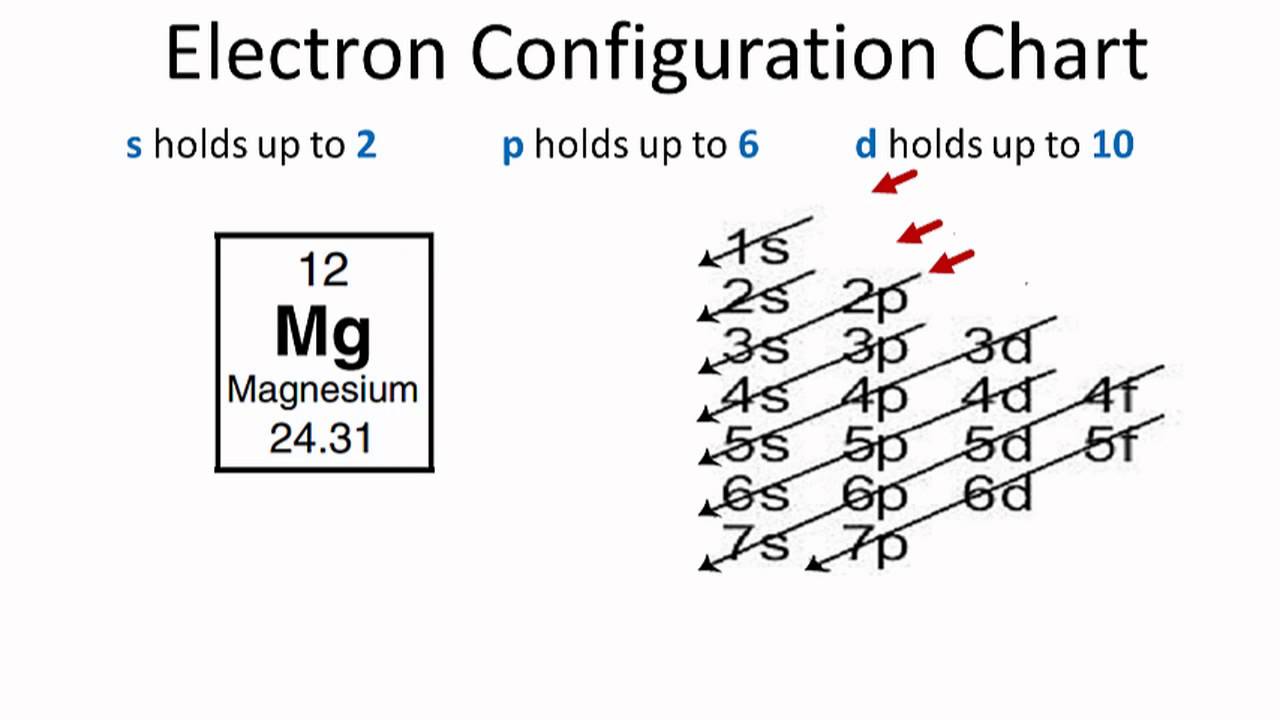
Magnesium Electron Configuration (Mg) with Orbital Diagram
The ground state electron configuration of ground state. Located in the iv period. The following table lists the ionization energies ie (ionization potentials);. Mn (manganese) is an element with position number 25 in the periodic table. Manganese atoms have 25 electrons and the shell structure is 2.8.13.2.
Manganese Properties Electron Configuration Vector Illustration Stock
The complete electron configuration for manganese should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d5 and the abbreviated electron. Manganese atoms have 25 electrons and the shell structure is 2.8.13.2. The ground state electron configuration of ground state. Mn (manganese) is an element with position number 25 in the periodic table. Calculate the effective nuclear charge of the.
Full Electron Configuration Of Manganese
Calculate the effective nuclear charge of the last electron in an atom whose configuration is 1s22s22p63s23p5. The following table lists the ionization energies ie (ionization potentials);. Located in the iv period. Manganese atoms have 25 electrons and the shell structure is 2.8.13.2. The full electron configuration of this element is 1s2 2s2 2p6 3s2 3p6 3d5 4s2, while its shortened.
Electron Configuration for Manganese (Mn2+, Mn3+, Mn4+ ions)
The following table lists the ionization energies ie (ionization potentials);. Located in the iv period. The ground state electron configuration of ground state. Mn (manganese) is an element with position number 25 in the periodic table. Calculate the effective nuclear charge of the last electron in an atom whose configuration is 1s22s22p63s23p5.
Electron Configuration Of Manganese Long Form
Shorthand electron configuration of manganese: The complete electron configuration for manganese should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d5 and the abbreviated electron. Calculate the effective nuclear charge of the last electron in an atom whose configuration is 1s22s22p63s23p5. The following table lists the ionization energies ie (ionization potentials);. The full electron configuration of this element is.
Electron Configuration For Manganese Illustrations, RoyaltyFree Vector
The complete electron configuration for manganese should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d5 and the abbreviated electron. Manganese atoms have 25 electrons and the shell structure is 2.8.13.2. The following table lists the ionization energies ie (ionization potentials);. Shorthand electron configuration of manganese: Located in the iv period.
Manganese Protons Neutrons Electrons Electron Configuration
Shorthand electron configuration of manganese: The ground state electron configuration of ground state. The complete electron configuration for manganese should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d5 and the abbreviated electron. Located in the iv period. Calculate the effective nuclear charge of the last electron in an atom whose configuration is 1s22s22p63s23p5.
Electron Configuration Of Manganese Long Form
Manganese atoms have 25 electrons and the shell structure is 2.8.13.2. Shorthand electron configuration of manganese: The following table lists the ionization energies ie (ionization potentials);. The full electron configuration of this element is 1s2 2s2 2p6 3s2 3p6 3d5 4s2, while its shortened. Located in the iv period.
Manganese Electron Configuration Dynamic Periodic Table of Elements
Manganese atoms have 25 electrons and the shell structure is 2.8.13.2. Shorthand electron configuration of manganese: The following table lists the ionization energies ie (ionization potentials);. The complete electron configuration for manganese should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d5 and the abbreviated electron. Located in the iv period.
Draw The Electron Configuration For A Neutral Atom Of Manganese
The following table lists the ionization energies ie (ionization potentials);. Shorthand electron configuration of manganese: Calculate the effective nuclear charge of the last electron in an atom whose configuration is 1s22s22p63s23p5. Located in the iv period. Manganese atoms have 25 electrons and the shell structure is 2.8.13.2.
Mn (Manganese) Is An Element With Position Number 25 In The Periodic Table.
The following table lists the ionization energies ie (ionization potentials);. Located in the iv period. The full electron configuration of this element is 1s2 2s2 2p6 3s2 3p6 3d5 4s2, while its shortened. The ground state electron configuration of ground state.
Manganese Atoms Have 25 Electrons And The Shell Structure Is 2.8.13.2.
Calculate the effective nuclear charge of the last electron in an atom whose configuration is 1s22s22p63s23p5. The complete electron configuration for manganese should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d5 and the abbreviated electron. Shorthand electron configuration of manganese: