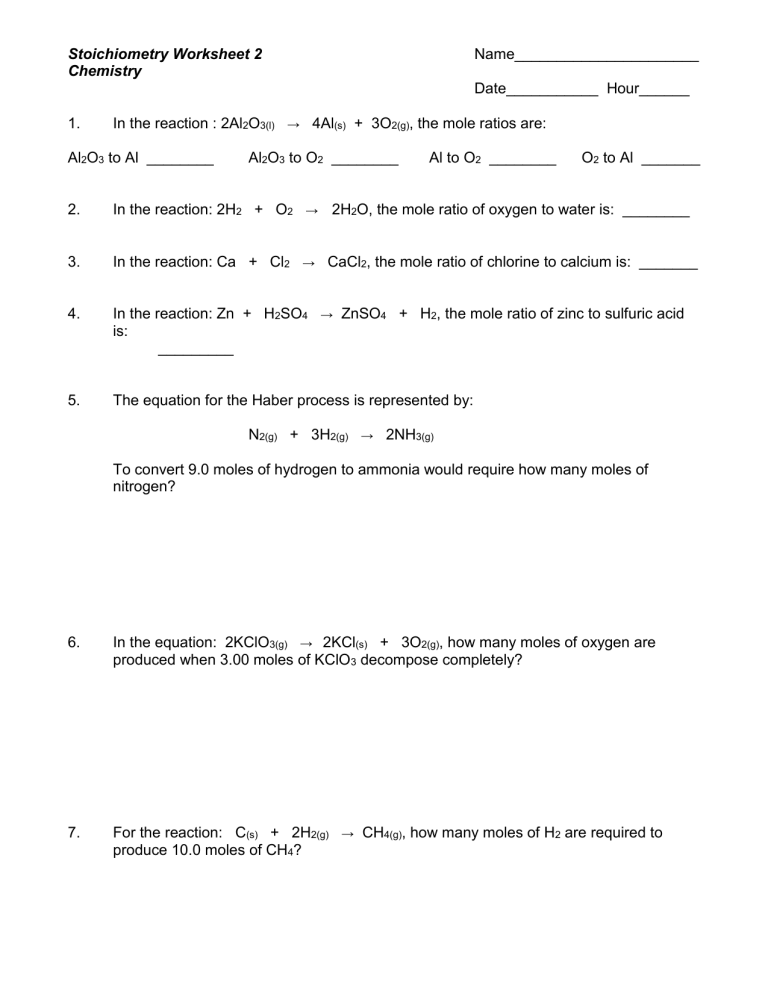
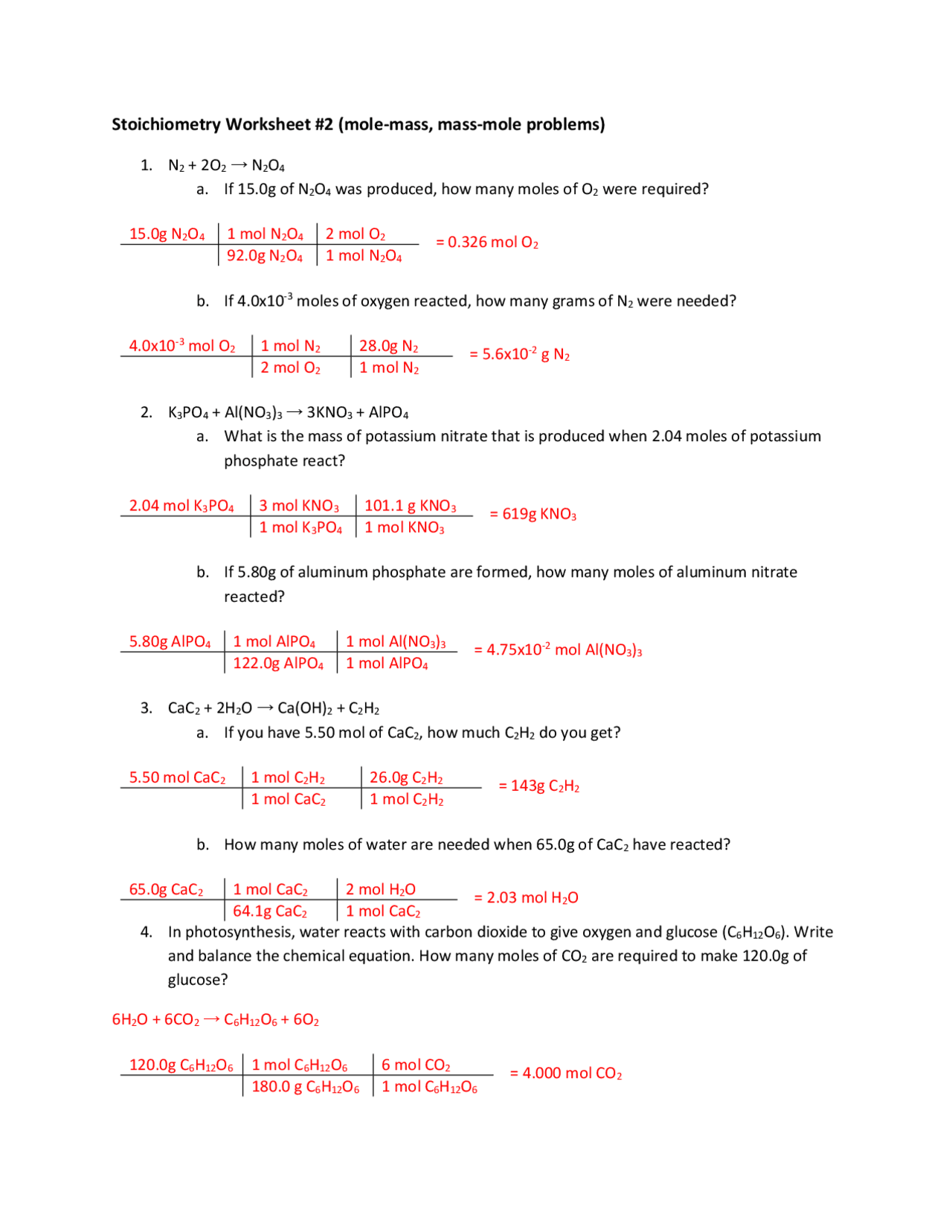
Chapter 9 Review Stoichiometry Section 1 Answer Key - Which chemical is in excess? Chapter 9 complete stoichiometry review practice problems with answer key.doc. For example, in the previous equation, the mole ratio of h₂ to o₂ is 2:1, meaning two moles of. By reviewing the answer key for section 1 of chapter 9, we can reinforce our understanding.
For example, in the previous equation, the mole ratio of h₂ to o₂ is 2:1, meaning two moles of. By reviewing the answer key for section 1 of chapter 9, we can reinforce our understanding. Chapter 9 complete stoichiometry review practice problems with answer key.doc. Which chemical is in excess?
For example, in the previous equation, the mole ratio of h₂ to o₂ is 2:1, meaning two moles of. Which chemical is in excess? Chapter 9 complete stoichiometry review practice problems with answer key.doc. By reviewing the answer key for section 1 of chapter 9, we can reinforce our understanding.
Answer Key
By reviewing the answer key for section 1 of chapter 9, we can reinforce our understanding. Chapter 9 complete stoichiometry review practice problems with answer key.doc. Which chemical is in excess? For example, in the previous equation, the mole ratio of h₂ to o₂ is 2:1, meaning two moles of.
Stoichiometry Worksheet Answer Key Unique Chemistry 12 Mr Nguyen S
Chapter 9 complete stoichiometry review practice problems with answer key.doc. By reviewing the answer key for section 1 of chapter 9, we can reinforce our understanding. Which chemical is in excess? For example, in the previous equation, the mole ratio of h₂ to o₂ is 2:1, meaning two moles of.
Stoichiometry Worksheets 1 Mole To Mole Calculations Answer
Which chemical is in excess? By reviewing the answer key for section 1 of chapter 9, we can reinforce our understanding. For example, in the previous equation, the mole ratio of h₂ to o₂ is 2:1, meaning two moles of. Chapter 9 complete stoichiometry review practice problems with answer key.doc.
Answer Key Stoichiometry Worksheets
Chapter 9 complete stoichiometry review practice problems with answer key.doc. Which chemical is in excess? For example, in the previous equation, the mole ratio of h₂ to o₂ is 2:1, meaning two moles of. By reviewing the answer key for section 1 of chapter 9, we can reinforce our understanding.
Answer Key Stoichiometry Worksheet
Which chemical is in excess? For example, in the previous equation, the mole ratio of h₂ to o₂ is 2:1, meaning two moles of. By reviewing the answer key for section 1 of chapter 9, we can reinforce our understanding. Chapter 9 complete stoichiometry review practice problems with answer key.doc.
Stoichiometry Worksheet Answer Key
Which chemical is in excess? For example, in the previous equation, the mole ratio of h₂ to o₂ is 2:1, meaning two moles of. By reviewing the answer key for section 1 of chapter 9, we can reinforce our understanding. Chapter 9 complete stoichiometry review practice problems with answer key.doc.
Mastering Stoichiometry Chapter 9 Review, Section 1 Answer Key
Which chemical is in excess? Chapter 9 complete stoichiometry review practice problems with answer key.doc. By reviewing the answer key for section 1 of chapter 9, we can reinforce our understanding. For example, in the previous equation, the mole ratio of h₂ to o₂ is 2:1, meaning two moles of.
The Ultimate Guide to Understanding Chapter 9 Review Stoichiometry
Chapter 9 complete stoichiometry review practice problems with answer key.doc. For example, in the previous equation, the mole ratio of h₂ to o₂ is 2:1, meaning two moles of. By reviewing the answer key for section 1 of chapter 9, we can reinforce our understanding. Which chemical is in excess?
Unit 08 Stoichiometry Worksheet 1 With Answer Key Download
Chapter 9 complete stoichiometry review practice problems with answer key.doc. For example, in the previous equation, the mole ratio of h₂ to o₂ is 2:1, meaning two moles of. Which chemical is in excess? By reviewing the answer key for section 1 of chapter 9, we can reinforce our understanding.
Chapter 9 Stoichiometry Chapter Review Answers
Chapter 9 complete stoichiometry review practice problems with answer key.doc. Which chemical is in excess? By reviewing the answer key for section 1 of chapter 9, we can reinforce our understanding. For example, in the previous equation, the mole ratio of h₂ to o₂ is 2:1, meaning two moles of.
Chapter 9 Complete Stoichiometry Review Practice Problems With Answer Key.doc.
Which chemical is in excess? By reviewing the answer key for section 1 of chapter 9, we can reinforce our understanding. For example, in the previous equation, the mole ratio of h₂ to o₂ is 2:1, meaning two moles of.