Chapter 8 Covalent Bonding - Compare the strengths of intermolecular attractions to the strengths of ionic bonds and. •a chemical bond formed by sharing a pair of electrons is called a covalent bond. Study with quizlet and memorize flashcards containing terms like when sharing of electrons. Halogens are an example of atoms that form diatomic molecules with a single covalent. A molecule is formed when two or more atoms bond covalently. Hybrid orbitals result from the mixing of atomic orbitals to hold lone electron pairs and.
Study with quizlet and memorize flashcards containing terms like when sharing of electrons. •a chemical bond formed by sharing a pair of electrons is called a covalent bond. A molecule is formed when two or more atoms bond covalently. Compare the strengths of intermolecular attractions to the strengths of ionic bonds and. Hybrid orbitals result from the mixing of atomic orbitals to hold lone electron pairs and. Halogens are an example of atoms that form diatomic molecules with a single covalent.
Halogens are an example of atoms that form diatomic molecules with a single covalent. Compare the strengths of intermolecular attractions to the strengths of ionic bonds and. A molecule is formed when two or more atoms bond covalently. •a chemical bond formed by sharing a pair of electrons is called a covalent bond. Hybrid orbitals result from the mixing of atomic orbitals to hold lone electron pairs and. Study with quizlet and memorize flashcards containing terms like when sharing of electrons.
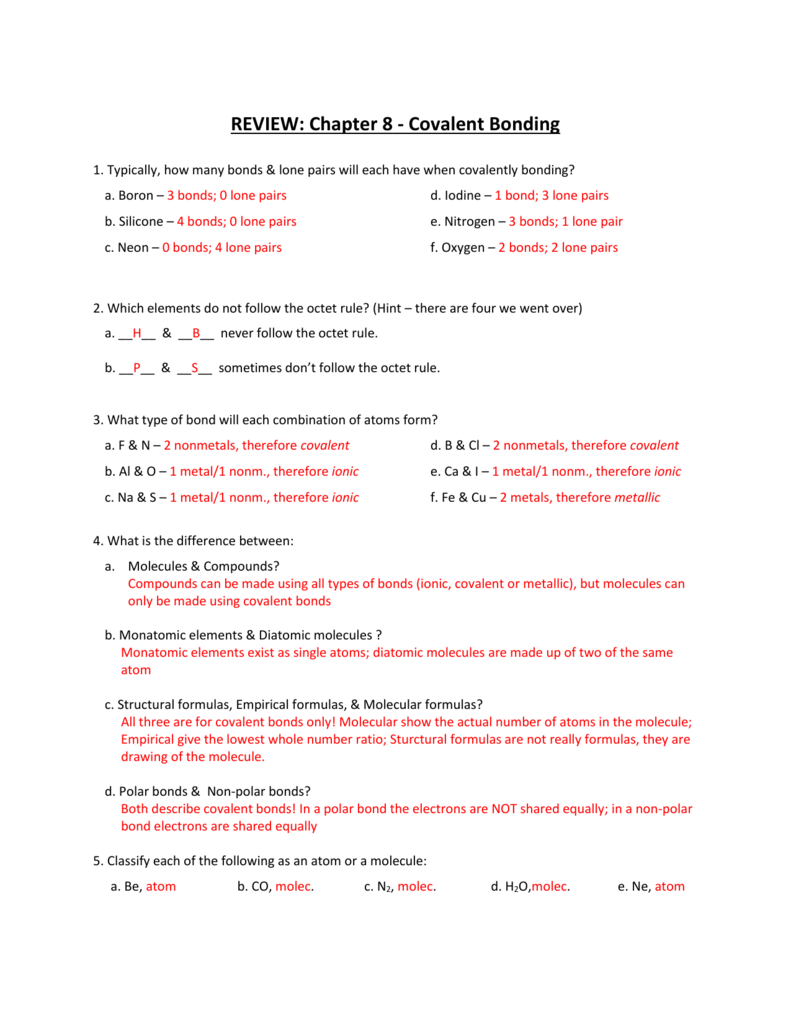
REVIEW Chapter 8 Covalent Bonding
Halogens are an example of atoms that form diatomic molecules with a single covalent. Compare the strengths of intermolecular attractions to the strengths of ionic bonds and. Hybrid orbitals result from the mixing of atomic orbitals to hold lone electron pairs and. A molecule is formed when two or more atoms bond covalently. •a chemical bond formed by sharing a.
Chapter 8 Covalent Bonding
Hybrid orbitals result from the mixing of atomic orbitals to hold lone electron pairs and. Study with quizlet and memorize flashcards containing terms like when sharing of electrons. Halogens are an example of atoms that form diatomic molecules with a single covalent. Compare the strengths of intermolecular attractions to the strengths of ionic bonds and. A molecule is formed when.
Chapter 8 Covalent Bonding
Compare the strengths of intermolecular attractions to the strengths of ionic bonds and. A molecule is formed when two or more atoms bond covalently. Halogens are an example of atoms that form diatomic molecules with a single covalent. Study with quizlet and memorize flashcards containing terms like when sharing of electrons. •a chemical bond formed by sharing a pair of.
The Ultimate Guide to Chapter 8 Covalent Bonding Worksheet Answers
Compare the strengths of intermolecular attractions to the strengths of ionic bonds and. Study with quizlet and memorize flashcards containing terms like when sharing of electrons. Hybrid orbitals result from the mixing of atomic orbitals to hold lone electron pairs and. A molecule is formed when two or more atoms bond covalently. Halogens are an example of atoms that form.
PPT Chapter 8 “Covalent Bonding” PowerPoint Presentation, free
A molecule is formed when two or more atoms bond covalently. Compare the strengths of intermolecular attractions to the strengths of ionic bonds and. •a chemical bond formed by sharing a pair of electrons is called a covalent bond. Halogens are an example of atoms that form diatomic molecules with a single covalent. Hybrid orbitals result from the mixing of.
PPT Covalent Bonding Chapter 8 PowerPoint Presentation, free download
Halogens are an example of atoms that form diatomic molecules with a single covalent. Compare the strengths of intermolecular attractions to the strengths of ionic bonds and. Hybrid orbitals result from the mixing of atomic orbitals to hold lone electron pairs and. A molecule is formed when two or more atoms bond covalently. •a chemical bond formed by sharing a.
Chapter 8. Covalent Bonding PDF Chemical Bond Covalent Bond
Compare the strengths of intermolecular attractions to the strengths of ionic bonds and. Study with quizlet and memorize flashcards containing terms like when sharing of electrons. Halogens are an example of atoms that form diatomic molecules with a single covalent. A molecule is formed when two or more atoms bond covalently. Hybrid orbitals result from the mixing of atomic orbitals.
Covalent Bonding Chapter 8
Compare the strengths of intermolecular attractions to the strengths of ionic bonds and. •a chemical bond formed by sharing a pair of electrons is called a covalent bond. A molecule is formed when two or more atoms bond covalently. Hybrid orbitals result from the mixing of atomic orbitals to hold lone electron pairs and. Study with quizlet and memorize flashcards.
Chemistry Chapter 8 Covalent Bonding Worksheet Answer Key
Halogens are an example of atoms that form diatomic molecules with a single covalent. •a chemical bond formed by sharing a pair of electrons is called a covalent bond. Study with quizlet and memorize flashcards containing terms like when sharing of electrons. Compare the strengths of intermolecular attractions to the strengths of ionic bonds and. Hybrid orbitals result from the.
30++ Covalent Bonding Worksheet Worksheets Decoomo
Hybrid orbitals result from the mixing of atomic orbitals to hold lone electron pairs and. Study with quizlet and memorize flashcards containing terms like when sharing of electrons. •a chemical bond formed by sharing a pair of electrons is called a covalent bond. Compare the strengths of intermolecular attractions to the strengths of ionic bonds and. A molecule is formed.
Study With Quizlet And Memorize Flashcards Containing Terms Like When Sharing Of Electrons.
A molecule is formed when two or more atoms bond covalently. •a chemical bond formed by sharing a pair of electrons is called a covalent bond. Hybrid orbitals result from the mixing of atomic orbitals to hold lone electron pairs and. Halogens are an example of atoms that form diatomic molecules with a single covalent.