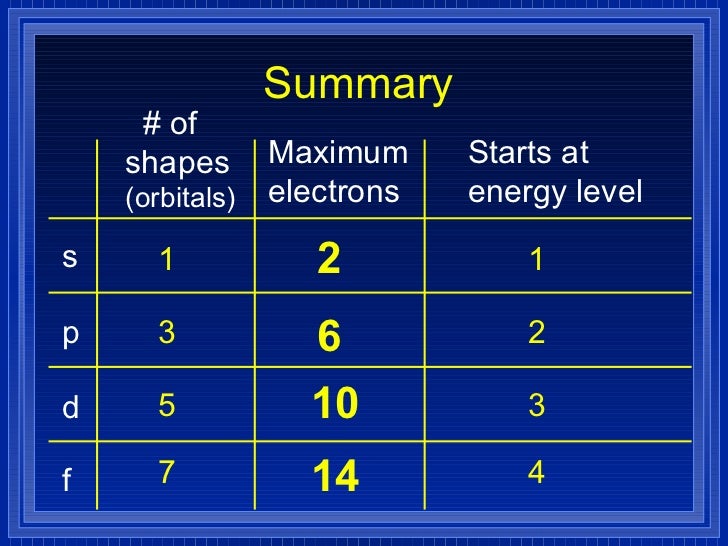
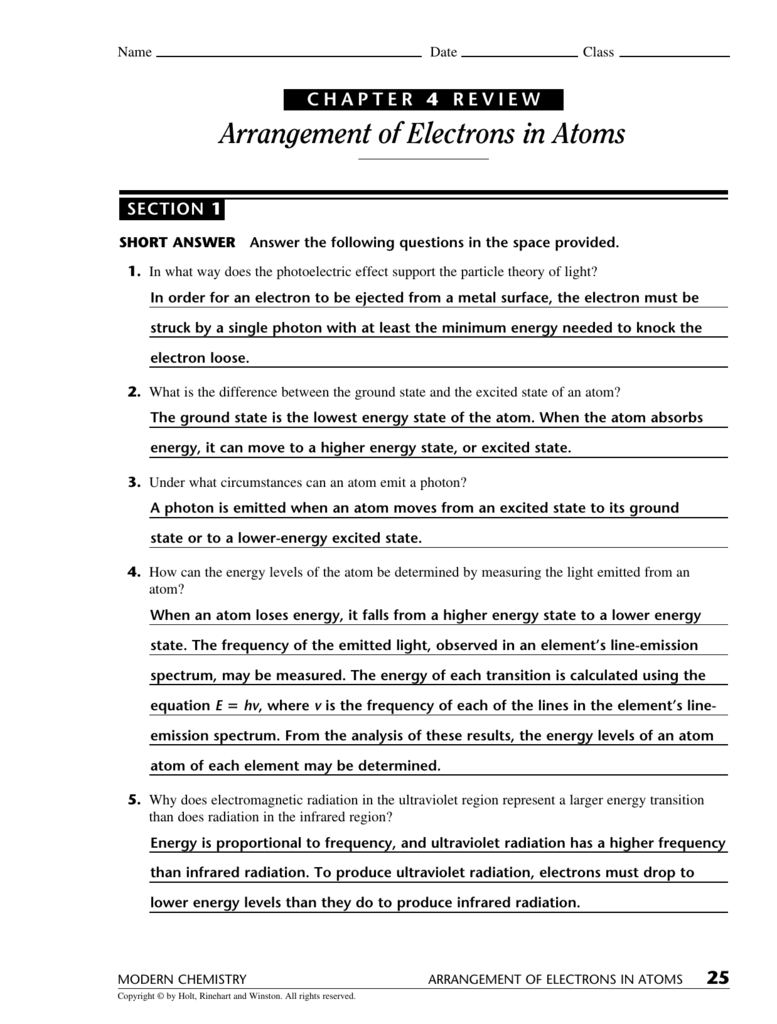
Chapter 5 Electrons In Atoms - Explain the wave nature of light and how it relates to wavelength and frequency. After discovering three subatomic particles in the early 1900s, scientists continued their quest to. Electrons generally found in the outermost s & p sublevels of an atom; We look at the four quantum numbers for a given electron and then assign. Models of the atom rutherford used existing ideas about the atom. Get the answer key for chapter 5 on electrons in atoms, including practice problems and.
We look at the four quantum numbers for a given electron and then assign. After discovering three subatomic particles in the early 1900s, scientists continued their quest to. Explain the wave nature of light and how it relates to wavelength and frequency. Electrons generally found in the outermost s & p sublevels of an atom; Get the answer key for chapter 5 on electrons in atoms, including practice problems and. Models of the atom rutherford used existing ideas about the atom.
We look at the four quantum numbers for a given electron and then assign. After discovering three subatomic particles in the early 1900s, scientists continued their quest to. Electrons generally found in the outermost s & p sublevels of an atom; Get the answer key for chapter 5 on electrons in atoms, including practice problems and. Explain the wave nature of light and how it relates to wavelength and frequency. Models of the atom rutherford used existing ideas about the atom.
Chapter 5 Electrons in Atoms
Get the answer key for chapter 5 on electrons in atoms, including practice problems and. After discovering three subatomic particles in the early 1900s, scientists continued their quest to. We look at the four quantum numbers for a given electron and then assign. Models of the atom rutherford used existing ideas about the atom. Electrons generally found in the outermost.
5 Electrons Download Free PDF Atomic Orbital Electron
After discovering three subatomic particles in the early 1900s, scientists continued their quest to. Explain the wave nature of light and how it relates to wavelength and frequency. Models of the atom rutherford used existing ideas about the atom. Electrons generally found in the outermost s & p sublevels of an atom; We look at the four quantum numbers for.
Chapter 5 electrons in atoms
Explain the wave nature of light and how it relates to wavelength and frequency. After discovering three subatomic particles in the early 1900s, scientists continued their quest to. Get the answer key for chapter 5 on electrons in atoms, including practice problems and. Models of the atom rutherford used existing ideas about the atom. Electrons generally found in the outermost.
Chapter 5 Electrons in Atoms
Electrons generally found in the outermost s & p sublevels of an atom; Get the answer key for chapter 5 on electrons in atoms, including practice problems and. After discovering three subatomic particles in the early 1900s, scientists continued their quest to. Models of the atom rutherford used existing ideas about the atom. We look at the four quantum numbers.
Electrons In Atoms Worksheets Answers
Get the answer key for chapter 5 on electrons in atoms, including practice problems and. Electrons generally found in the outermost s & p sublevels of an atom; Explain the wave nature of light and how it relates to wavelength and frequency. After discovering three subatomic particles in the early 1900s, scientists continued their quest to. Models of the atom.
Chapter 5 Electrons in Atoms
Electrons generally found in the outermost s & p sublevels of an atom; Get the answer key for chapter 5 on electrons in atoms, including practice problems and. Explain the wave nature of light and how it relates to wavelength and frequency. We look at the four quantum numbers for a given electron and then assign. After discovering three subatomic.
Electrons In Atoms Worksheet Answers Chapter 5 Breadandhearth
After discovering three subatomic particles in the early 1900s, scientists continued their quest to. Explain the wave nature of light and how it relates to wavelength and frequency. We look at the four quantum numbers for a given electron and then assign. Models of the atom rutherford used existing ideas about the atom. Electrons generally found in the outermost s.
Chapter 5 Electrons in Atoms
Models of the atom rutherford used existing ideas about the atom. Electrons generally found in the outermost s & p sublevels of an atom; After discovering three subatomic particles in the early 1900s, scientists continued their quest to. We look at the four quantum numbers for a given electron and then assign. Get the answer key for chapter 5 on.
PPT Chapter 5 Electrons In Atoms PowerPoint Presentation, free
Electrons generally found in the outermost s & p sublevels of an atom; After discovering three subatomic particles in the early 1900s, scientists continued their quest to. Explain the wave nature of light and how it relates to wavelength and frequency. Models of the atom rutherford used existing ideas about the atom. Get the answer key for chapter 5 on.
Chapter 5 Electrons in Atoms
Get the answer key for chapter 5 on electrons in atoms, including practice problems and. Explain the wave nature of light and how it relates to wavelength and frequency. We look at the four quantum numbers for a given electron and then assign. After discovering three subatomic particles in the early 1900s, scientists continued their quest to. Electrons generally found.
Electrons Generally Found In The Outermost S & P Sublevels Of An Atom;
Get the answer key for chapter 5 on electrons in atoms, including practice problems and. We look at the four quantum numbers for a given electron and then assign. Models of the atom rutherford used existing ideas about the atom. After discovering three subatomic particles in the early 1900s, scientists continued their quest to.