Chapter 4 Test Arrangement Of Electrons In Atoms Answer Key - What are the possible angular momentum quantum #'s?, what is the total # of electrons. De broglie suggested that electrons should be considered as waves confined to the space. Separating unpaired electrons into as many orbitals as possible. The development of a new atomi in the. Class arrangement of electrons in ato s section quiz: How many electrons are needed to occupy an energy level? What are the rules that govern. Wave b has the higher frequency.
What are the possible angular momentum quantum #'s?, what is the total # of electrons. How many electrons are needed to occupy an energy level? Wave b has the higher frequency. The development of a new atomi in the. De broglie suggested that electrons should be considered as waves confined to the space. Class arrangement of electrons in ato s section quiz: What are the rules that govern. Separating unpaired electrons into as many orbitals as possible.
What are the possible angular momentum quantum #'s?, what is the total # of electrons. De broglie suggested that electrons should be considered as waves confined to the space. Class arrangement of electrons in ato s section quiz: Separating unpaired electrons into as many orbitals as possible. What are the rules that govern. How many electrons are needed to occupy an energy level? The development of a new atomi in the. Wave b has the higher frequency.
The Essential Guide to Understanding Electron Arrangement in Atoms
Separating unpaired electrons into as many orbitals as possible. Class arrangement of electrons in ato s section quiz: What are the rules that govern. How many electrons are needed to occupy an energy level? Wave b has the higher frequency.
Unlocking the Secrets of Electrons in Atoms Chapter 4 Answer Key Revealed
What are the rules that govern. The development of a new atomi in the. Separating unpaired electrons into as many orbitals as possible. Class arrangement of electrons in ato s section quiz: Wave b has the higher frequency.
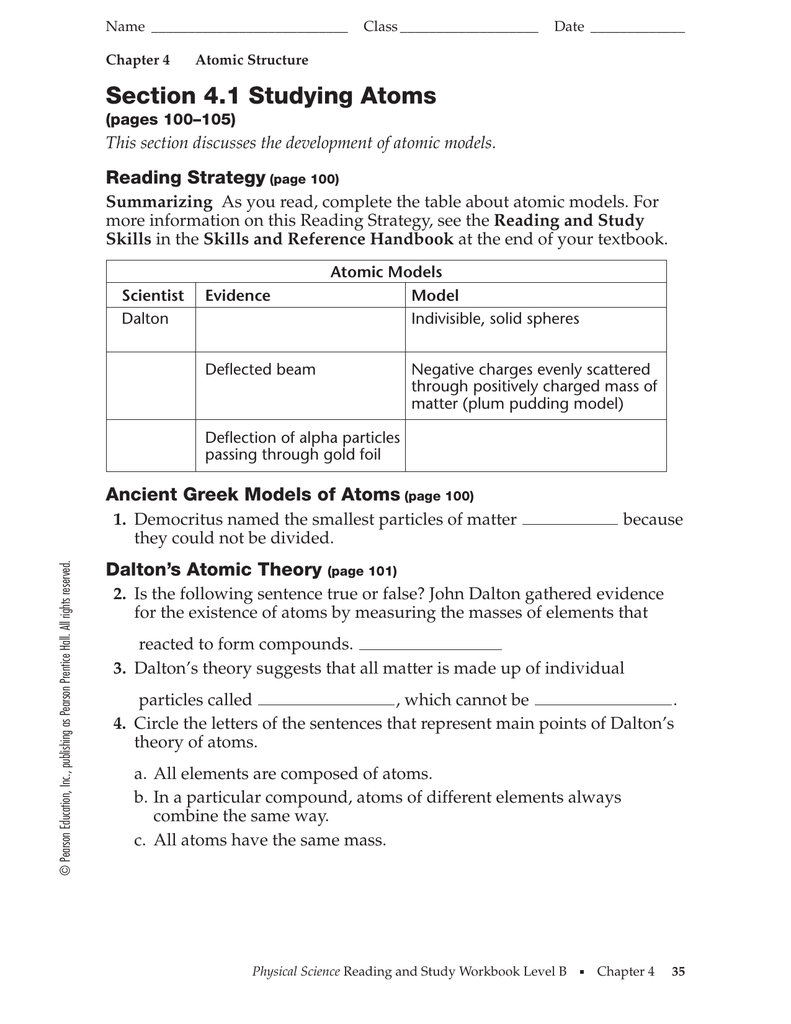
SECTION 4.1 STUDYING ATOMS ANSWER KEY
Class arrangement of electrons in ato s section quiz: De broglie suggested that electrons should be considered as waves confined to the space. What are the possible angular momentum quantum #'s?, what is the total # of electrons. What are the rules that govern. Separating unpaired electrons into as many orbitals as possible.
Unlocking the Secrets of Electrons in Atoms Chapter 4 Answer Key Revealed
How many electrons are needed to occupy an energy level? What are the possible angular momentum quantum #'s?, what is the total # of electrons. Class arrangement of electrons in ato s section quiz: Separating unpaired electrons into as many orbitals as possible. Wave b has the higher frequency.
The Essential Guide to Understanding Electron Arrangement in Atoms
What are the possible angular momentum quantum #'s?, what is the total # of electrons. What are the rules that govern. The development of a new atomi in the. Wave b has the higher frequency. De broglie suggested that electrons should be considered as waves confined to the space.
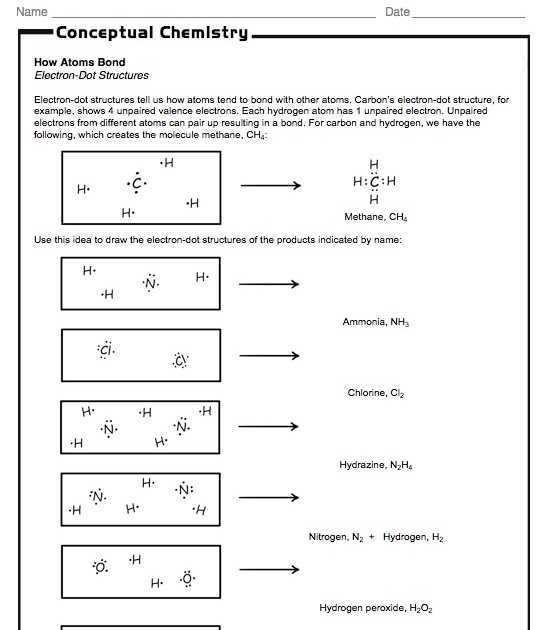
Worksheets Electrons In Atoms Answer Key
De broglie suggested that electrons should be considered as waves confined to the space. What are the rules that govern. Separating unpaired electrons into as many orbitals as possible. Wave b has the higher frequency. How many electrons are needed to occupy an energy level?
10+ Chapter 5.1 Electrons In Atoms Answer Key LannaBallena
What are the possible angular momentum quantum #'s?, what is the total # of electrons. De broglie suggested that electrons should be considered as waves confined to the space. The development of a new atomi in the. What are the rules that govern. How many electrons are needed to occupy an energy level?
Unveiling the Atomic Structure Unraveling the Answer Key to Chapter 4
The development of a new atomi in the. How many electrons are needed to occupy an energy level? Wave b has the higher frequency. De broglie suggested that electrons should be considered as waves confined to the space. What are the possible angular momentum quantum #'s?, what is the total # of electrons.
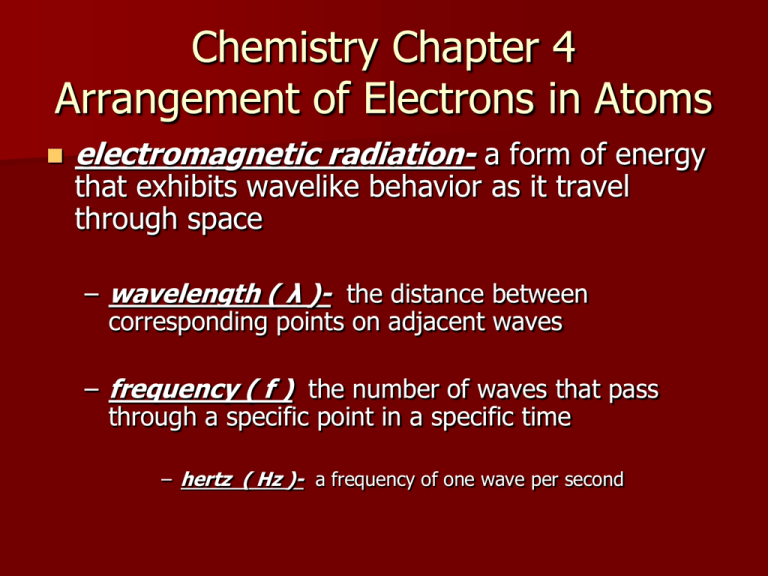
Chemistry Chapter 4 Arrangement of Electrons in Atoms
How many electrons are needed to occupy an energy level? What are the possible angular momentum quantum #'s?, what is the total # of electrons. Wave b has the higher frequency. What are the rules that govern. The development of a new atomi in the.
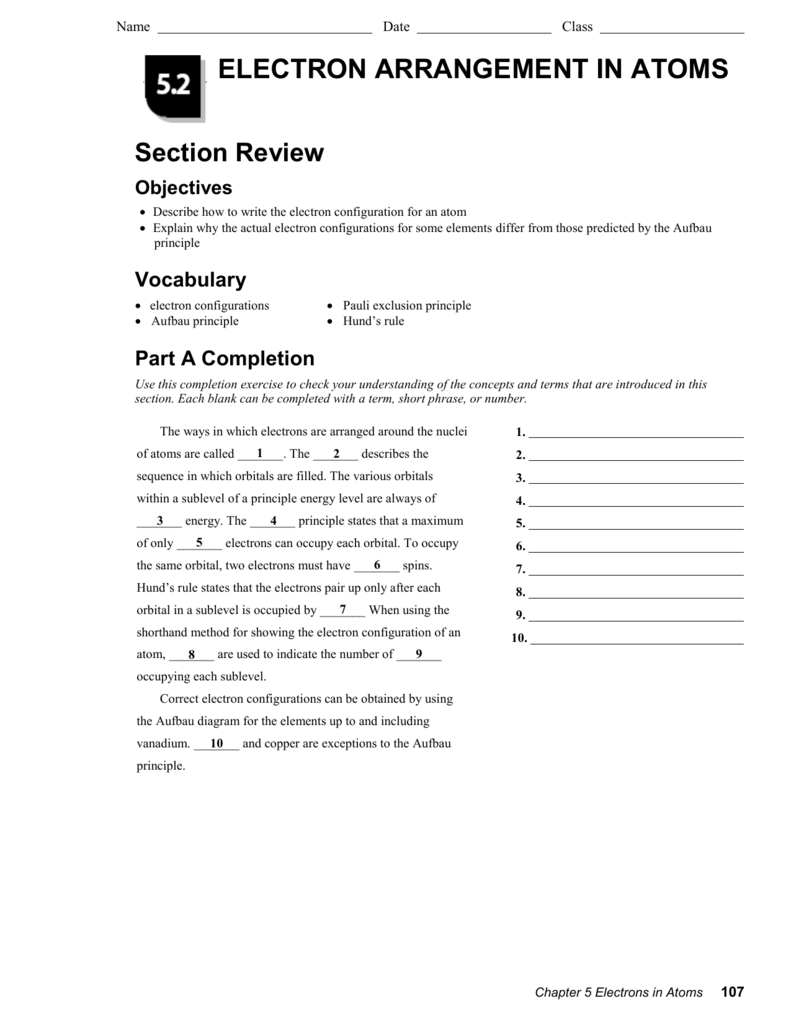
Chapter 5 Electrons In Atoms Answers To Worksheet Ivuyteq
Class arrangement of electrons in ato s section quiz: The development of a new atomi in the. De broglie suggested that electrons should be considered as waves confined to the space. What are the possible angular momentum quantum #'s?, what is the total # of electrons. What are the rules that govern.
Separating Unpaired Electrons Into As Many Orbitals As Possible.
Class arrangement of electrons in ato s section quiz: What are the possible angular momentum quantum #'s?, what is the total # of electrons. Wave b has the higher frequency. De broglie suggested that electrons should be considered as waves confined to the space.
What Are The Rules That Govern.
The development of a new atomi in the. How many electrons are needed to occupy an energy level?