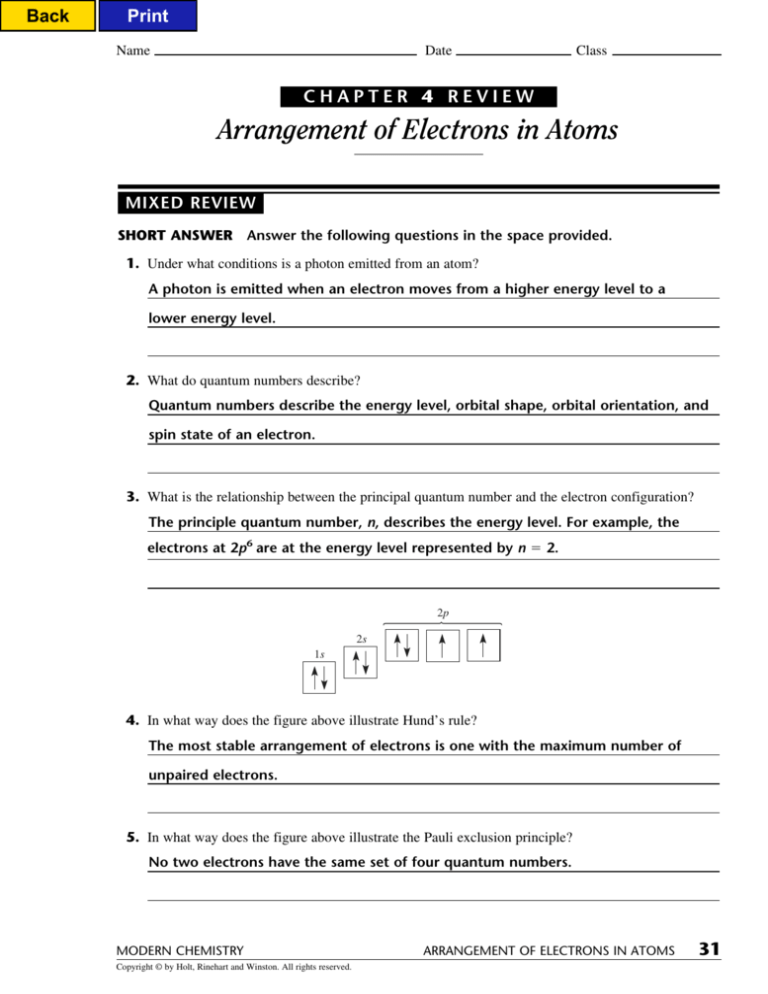
Chapter 4 Review Arrangement Of Electrons In Atoms - Test your knowledge of the development of atomic models, the quantum model of the atom,. Study with quizlet and memorize flashcards containing terms like how many quantum. Orbitals of equal energy are each occupied by one electron before any orbital is occupied by a. To produce ultraviolet radiation, electrons must drop to lower energy levels than they do to.
Orbitals of equal energy are each occupied by one electron before any orbital is occupied by a. Test your knowledge of the development of atomic models, the quantum model of the atom,. To produce ultraviolet radiation, electrons must drop to lower energy levels than they do to. Study with quizlet and memorize flashcards containing terms like how many quantum.
Orbitals of equal energy are each occupied by one electron before any orbital is occupied by a. Study with quizlet and memorize flashcards containing terms like how many quantum. To produce ultraviolet radiation, electrons must drop to lower energy levels than they do to. Test your knowledge of the development of atomic models, the quantum model of the atom,.
Unveiling the Atomic Structure Unraveling the Answer Key to Chapter 4
Study with quizlet and memorize flashcards containing terms like how many quantum. Orbitals of equal energy are each occupied by one electron before any orbital is occupied by a. To produce ultraviolet radiation, electrons must drop to lower energy levels than they do to. Test your knowledge of the development of atomic models, the quantum model of the atom,.
Ch 4 Arrangement of Electrons in Atoms Mrs. Bolden's Chemistry Site
Study with quizlet and memorize flashcards containing terms like how many quantum. Test your knowledge of the development of atomic models, the quantum model of the atom,. To produce ultraviolet radiation, electrons must drop to lower energy levels than they do to. Orbitals of equal energy are each occupied by one electron before any orbital is occupied by a.
Chapter 4 Review Arrangement Of Electrons In Atoms Answer Key
Orbitals of equal energy are each occupied by one electron before any orbital is occupied by a. Test your knowledge of the development of atomic models, the quantum model of the atom,. To produce ultraviolet radiation, electrons must drop to lower energy levels than they do to. Study with quizlet and memorize flashcards containing terms like how many quantum.
Chapter 4 Arrangement of Electrons in Atoms Diagram Quizlet
To produce ultraviolet radiation, electrons must drop to lower energy levels than they do to. Study with quizlet and memorize flashcards containing terms like how many quantum. Test your knowledge of the development of atomic models, the quantum model of the atom,. Orbitals of equal energy are each occupied by one electron before any orbital is occupied by a.
Chapter 4 Review Sheet PDF
Orbitals of equal energy are each occupied by one electron before any orbital is occupied by a. To produce ultraviolet radiation, electrons must drop to lower energy levels than they do to. Study with quizlet and memorize flashcards containing terms like how many quantum. Test your knowledge of the development of atomic models, the quantum model of the atom,.
Mastering Electron Arrangements Chapter 4 Review EXPERT Tips
Orbitals of equal energy are each occupied by one electron before any orbital is occupied by a. Study with quizlet and memorize flashcards containing terms like how many quantum. Test your knowledge of the development of atomic models, the quantum model of the atom,. To produce ultraviolet radiation, electrons must drop to lower energy levels than they do to.
Chemistry Chapter 4 Arrangement of Electrons in Atoms
Test your knowledge of the development of atomic models, the quantum model of the atom,. Study with quizlet and memorize flashcards containing terms like how many quantum. Orbitals of equal energy are each occupied by one electron before any orbital is occupied by a. To produce ultraviolet radiation, electrons must drop to lower energy levels than they do to.
Chapter 4 Arrangement of Electrons in Atoms I
Orbitals of equal energy are each occupied by one electron before any orbital is occupied by a. Test your knowledge of the development of atomic models, the quantum model of the atom,. Study with quizlet and memorize flashcards containing terms like how many quantum. To produce ultraviolet radiation, electrons must drop to lower energy levels than they do to.
Unveiling the Atomic Structure Unraveling the Answer Key to Chapter 4
To produce ultraviolet radiation, electrons must drop to lower energy levels than they do to. Study with quizlet and memorize flashcards containing terms like how many quantum. Test your knowledge of the development of atomic models, the quantum model of the atom,. Orbitals of equal energy are each occupied by one electron before any orbital is occupied by a.
Chapter 4 Review Arrangement Of Electrons In Atoms Answer Key Islero
Orbitals of equal energy are each occupied by one electron before any orbital is occupied by a. To produce ultraviolet radiation, electrons must drop to lower energy levels than they do to. Study with quizlet and memorize flashcards containing terms like how many quantum. Test your knowledge of the development of atomic models, the quantum model of the atom,.
Study With Quizlet And Memorize Flashcards Containing Terms Like How Many Quantum.
Orbitals of equal energy are each occupied by one electron before any orbital is occupied by a. Test your knowledge of the development of atomic models, the quantum model of the atom,. To produce ultraviolet radiation, electrons must drop to lower energy levels than they do to.